The antiepileptic and ictogenic effects of optogenetic neurostimulation of PV-expressing interneurons
- PMID: 27486107
- PMCID: PMC5144715
- DOI: 10.1152/jn.00744.2015
The antiepileptic and ictogenic effects of optogenetic neurostimulation of PV-expressing interneurons
Abstract
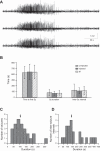
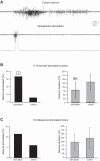
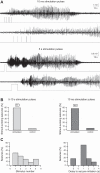
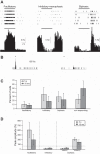
Parvalbumin (PV)-expressing interneurons exert powerful inhibitory effects on the normal cortical network; thus optogenetic activation of PV interneurons may also possess antiepileptic properties. To investigate this possibility we expressed channelrhodopsin 2 in PV interneurons by locally injecting the Cre-dependent viral vector AAV2/1-EF1a-DIO-ChETA-EYFP into the S1 barrel cortex of PV-Cre mice. Approximately 3-4 wk later recurrent electrographic seizures were evoked by local application of the chemoconvulsant 4-aminopyridine (4-AP); the ECoG and unit activity were monitored with extracellular silicone electrodes; and PV interneurons were activated optogenetically during the ictal and interictal phases. Five- to ten-second optogenetic activation of PV interneurons applied during electrographic seizures (ictal phase) terminated 33.7% of electrographic seizures compared with only 6% during sham stimulation, and the average electrographic seizure duration shortened by 38.7 ± 34.2% compared with sham stimulation. In contrast, interictal optogenetic activation of PV interneurons showed powerful and robust ictogenic effects. Approximately 60% of interictal optogenetic stimuli resulted in electrographic seizure initiation. Single-unit recordings revealed that presumptive PV-expressing interneurons markedly increased their firing during optogenetic stimulation, while many presumptive excitatory pyramidal neurons showed a biphasic response, with initial suppression of firing during the optogenetic pulse followed by a synchronized rebound increase in firing at the end of the laser pulse. Our findings indicated that ictal activation of PV-expressing interneurons possesses antiepileptic properties probably due to suppression of firing in pyramidal neurons during the laser pulse. However, in addition interictal activation of PV-expressing interneurons possesses powerful ictogenic properties, probably due to synchronized postinhibition rebound firing of pyramidal neurons.
Keywords: epilepsy; interneurons; neocortex; neurostimulation; optogenetics.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. - PubMed
-
- Bergey GK. Neurostimulation in the treatment of epilepsy. Exp Neurol 244: 87–95, 2013. - PubMed
-
- Berglind F, Ledri M, Sørensen AT, Nikitidou L, Melis M, Bielefeld P, Kirik D, Deisseroth K, Andersson M, Kokaia M. Optogenetic inhibition of chemically induced hypersynchronized bursting in mice. Neurobiol Dis 65: 133–141, 2014. - PubMed
-
- Blauwblomme T, Jiruska P, Huberfeld G. Mechanisms of ictogenesis. Int Rev Neurobiol 114: 155–185, 2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

