Amyloid-β Precursor Protein Modulates the Sorting of Testican-1 and Contributes to Its Accumulation in Brain Tissue and Cerebrospinal Fluid from Patients with Alzheimer Disease
- PMID: 27486134
- PMCID: PMC5015660
- DOI: 10.1093/jnen/nlw065
Amyloid-β Precursor Protein Modulates the Sorting of Testican-1 and Contributes to Its Accumulation in Brain Tissue and Cerebrospinal Fluid from Patients with Alzheimer Disease
Abstract
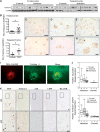
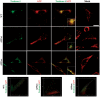
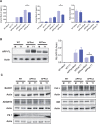
The mechanisms leading to amyloid-β (Aβ) accumulation in sporadic Alzheimer disease (AD) are unknown but both increased production or impaired clearance likely contribute to aggregation. To understand the potential roles of the extracellular matrix proteoglycan Testican-1 in the pathophysiology of AD, we used samples from AD patients and controls and an in vitro approach. Protein expression analysis showed increased levels of Testican-1 in frontal and temporal cortex of AD patients; histological analysis showed that Testican-1 accumulates and co-aggregates with Aβ plaques in the frontal, temporal and entorhinal cortices of AD patients. Proteomic analysis identified 10 fragments of Testican-1 in cerebrospinal fluid (CSF) from AD patients. HEK293T cells expressing human wild type or mutant Aβ precursor protein (APP) were transfected with Testican-1. The co-expression of both proteins modified the sorting of Testican-1 into the endocytic pathway leading to its transient accumulation in Golgi, which seemed to affect APP processing, as indicated by reduced Aβ40 and Aβ42 levels in APP mutant cells. In conclusion, patient data reflect a clearance impairment that may favor Aβ accumulation in AD brains and our in vitro model supports the notion that the interaction between APP and Testican-1 may be a key step in the production and aggregation of Aβ species.
Keywords: APP Swedish mutation; Alzheimer disease; Early endosomes; Neuritic plaques; Testican-1; β-Amyloid.
© 2016 Oxford University Press OR American Association of Neuropathologists.
Figures


Similar articles
-
Evaluation of the Expression of Amyloid Precursor Protein and the Ratio of Secreted Amyloid Beta 42 to Amyloid Beta 40 in SH-SY5Y Cells Stably Transfected with Wild-Type, Single-Mutant and Double-Mutant Forms of the APP Gene for the Study of Alzheimer's Disease Pathology.Appl Biochem Biotechnol. 2017 Nov;183(3):853-866. doi: 10.1007/s12010-017-2468-6. Epub 2017 Apr 17. Appl Biochem Biotechnol. 2017. PMID: 28417423
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412
-
Changes in amyloid-β and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein.Sci Transl Med. 2013 Jul 17;5(194):194re2. doi: 10.1126/scitranslmed.3006446. Sci Transl Med. 2013. PMID: 23863834
-
Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer's disease.Pathol Int. 2017 Apr;67(4):185-193. doi: 10.1111/pin.12520. Epub 2017 Mar 5. Pathol Int. 2017. PMID: 28261941 Review.
Cited by
-
CHRNA5 links chandelier cells to severity of amyloid pathology in aging and Alzheimer's disease.Transl Psychiatry. 2024 Feb 8;14(1):83. doi: 10.1038/s41398-024-02785-3. Transl Psychiatry. 2024. PMID: 38331937 Free PMC article.
-
Molecular signature of extracellular matrix pathology in schizophrenia.Eur J Neurosci. 2021 Jun;53(12):3960-3987. doi: 10.1111/ejn.15009. Epub 2020 Nov 13. Eur J Neurosci. 2021. PMID: 33070392 Free PMC article.
-
SPOCK1: a multi-domain proteoglycan at the crossroads of extracellular matrix remodeling and cancer development.Am J Cancer Res. 2020 Oct 1;10(10):3127-3137. eCollection 2020. Am J Cancer Res. 2020. PMID: 33163261 Free PMC article. Review.
-
Profiling of Serum Exosome MiRNA Reveals the Potential of a MiRNA Panel as Diagnostic Biomarker for Alzheimer's Disease.Mol Neurobiol. 2021 Jul;58(7):3084-3094. doi: 10.1007/s12035-021-02323-y. Epub 2021 Feb 24. Mol Neurobiol. 2021. PMID: 33629272
-
Nebulized inhalation of extracellular vesicles containing SPOCK2 suppresses lung adenocarcinoma progression via MAPK inhibition.Discov Oncol. 2025 May 17;16(1):797. doi: 10.1007/s12672-025-02626-9. Discov Oncol. 2025. PMID: 40382517 Free PMC article.
References
-
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med 2010;362:329–44 - PubMed
-
- Thal DR, Rüb U, Orantes M, et al. Phases of A -deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 - PubMed
-
- Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid -protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996;2:864–70 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

