Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients With ≥3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD
- PMID: 27486203
- PMCID: PMC5189616
- DOI: 10.1634/theoncologist.2016-0104
Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients With ≥3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD
Abstract
Background: This retrospective study evaluated the treatment patterns in and overall survival (OS) of multiple myeloma (MM) patients who were refractory to a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) or who had received three or more prior lines of therapy (LOTs) including a PI and an IMiD.
Methods: Electronic health records in the IMS LifeLink and OPTUM databases were screened for indexing periods of 2000-2014 and 2007-2014, respectively. Patients who were refractory to both a PI and an IMiD (criterion 1) or who received three or more prior LOTs (including a PI and an IMiD) and showed disease progression within 60 days of their most recent regimen (criterion 2) comprised the eligible population. Median OS from time of last LOT was assessed for the full cohort, cohorts meeting criteria 1 and 2, and clinically important subgroups.
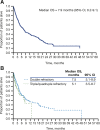
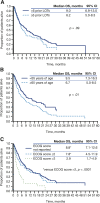
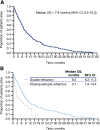
Results: Of 3,929 and 3,837 patients with MM diagnoses evaluated in the IMS LifeLink and OPTUM databases, 500 and 162 met the eligibility criteria, respectively. Similar median OS was observed for eligible patients in the IMS LifeLink and OPTUM databases (7.9 vs. 7.9 months; p = .5358). In subgroup analyses of the IMS LifeLink data set, median OS was longer in patients <65 years of age than it was for those ≥65 years at eligibility (9.5 vs 6.7 months; p < .01) and in patients with good or unreported versus poor performance status at last claim (7.8 or 8.8 vs. 2.9 months; p < .0001).
Conclusion: The findings of this survival analysis suggest that outcomes for these patients remain poor despite the availability of newer agents.
Implications for practice: This real-world retrospective study of electronic health records examines the survival outcomes of patients with multiple myeloma who are heavily pretreated or highly refractory to currently approved treatments, including recently approved proteasome inhibitors and immunomodulatory drugs. This survival analysis showed that outcomes for these patients remain poor despite the availability of newer agents, with median overall survival of approximately 8 months. These findings highlight a critical need to develop novel therapies for these patients and also serve as a reference point against which emerging agents for heavily pretreated or highly refractory disease may be evaluated.
Keywords: Electronic health records; Multiple myeloma; Retrospective studies; Survival analysis.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Harousseau JL. Ten years of improvement in the management of multiple myeloma: 2000–2010. Clin Lymphoma Myeloma Leuk. 2010;10:424–442. - PubMed
-
- Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23:1152–1157. - PubMed
-
- Meadows JP, Mark TM. Management of double-refractory multiple myeloma. Curr Hematol Malig Rep. 2013;8:253–260. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

