Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung
- PMID: 27486204
- PMCID: PMC5329050
- DOI: 10.1136/thoraxjnl-2016-208599
Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung
Abstract
Introduction: Azithromycin (AZM) reduces pulmonary inflammation and exacerbations in patients with COPD having emphysema. The antimicrobial effects of AZM on the lower airway microbiome are not known and may contribute to its beneficial effects. Here we tested whether AZM treatment affects the lung microbiome and bacterial metabolites that might contribute to changes in levels of inflammatory cytokines in the airways.
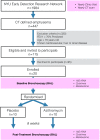
Methods: 20 smokers (current or ex-smokers) with emphysema were randomised to receive AZM 250 mg or placebo daily for 8 weeks. Bronchoalveolar lavage (BAL) was performed at baseline and after treatment. Measurements performed in acellular BAL fluid included 16S rRNA gene sequences and quantity; 39 cytokines, chemokines and growth factors and 119 identified metabolites. The response to lipopolysaccharide (LPS) by alveolar macrophages after ex-vivo treatment with AZM or bacterial metabolites was assessed.
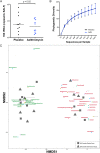
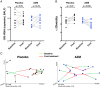
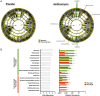
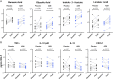
Results: Compared with placebo, AZM did not alter bacterial burden but reduced α-diversity, decreasing 11 low abundance taxa, none of which are classical pulmonary pathogens. Compared with placebo, AZM treatment led to reduced in-vivo levels of chemokine (C-X-C) ligand 1 (CXCL1), tumour necrosis factor (TNF)-α, interleukin (IL)-13 and IL-12p40 in BAL, but increased bacterial metabolites including glycolic acid, indol-3-acetate and linoleic acid. Glycolic acid and indol-3-acetate, but not AZM, blunted ex-vivo LPS-induced alveolar macrophage generation of CXCL1, TNF-α, IL-13 and IL-12p40.
Conclusion: AZM treatment altered both lung microbiota and metabolome, affecting anti-inflammatory bacterial metabolites that may contribute to its therapeutic effects.
Trial registration number: NCT02557958.
Keywords: Bronchoscopy; COPD ÀÜ Mechanisms.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures

Comment in
-
Macrolides, inflammation and the lung microbiome: untangling the web of causality.Thorax. 2017 Jan;72(1):10-12. doi: 10.1136/thoraxjnl-2016-209180. Epub 2016 Oct 31. Thorax. 2017. PMID: 27799630 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
