Pros and Cons of Adding of Neoadjuvant Chemotherapy to Standard Concurrent Chemoradiotherapy in Cervical Cancer: A Regional Cancer Center Experience
- PMID: 27486286
- PMCID: PMC4958073
- DOI: 10.1007/s13224-015-0698-5
Pros and Cons of Adding of Neoadjuvant Chemotherapy to Standard Concurrent Chemoradiotherapy in Cervical Cancer: A Regional Cancer Center Experience
Abstract
Background: The present study summarizes the results of treatment in the form of disease-free survival and overall survival in bulky stage IB2 and locally advanced (stages II-IVA) squamous cell carcinoma of the uterine cervix. The treatment has been given in the form of NACT followed by CCRT in one arm and CCRT in the other arm.
Materials and methods: This retrospective study analyzed 713 cervical cancer patients who were treated at our center during 2007 and 2008; out of 713 patients, data of 612 patients have been compared. The patients' data were analyzed retrospectively. Patients had undergone PF 28.6 %, TPF 21.5 %, and only CCRT 49.9 %. Majority of patients were in the age group 41-50 years, while stage wise, mainly stage IIIb and IIb. Disease-free survival was observed on the basis of stage and NACT. The survival analyses were performed using the Kaplan-Meier method. All statistical calculations were done with SPSS Statistics version 20.0.
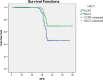
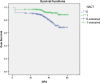
Results: For cancer cervix NACT versus CCRT, the DFS rate was at 5 years (58.3 vs. 41.8 % p = 0.001). NACT followed by CCRT demonstrated significantly superior DFS as compared to definitive CCRT, respectively, TPF (hazard ratio (HR) = 0.248, 95 % confidence interval (CI) 0.123-0.500; p < 0.001), PF (HR = 0.445, 95 % CI 0.266-0.722; p = 0.002). The results of univariate stage, age, and multivariate study show that stage hemoglobin level, interval between external-intracavitary radiation, and type of neoadjuvant chemotherapy were the factors affected survival cervical patients treated with radiation. The grade 3/4 hematologic toxicities were more in the NACT group than CCRT (p < 0.001) while the non-hematological toxicity was not significant; the TPF group experienced more toxicity than PF (p = 0.029). This treatment regimen is feasible as evidenced by the acceptable toxicity of NACT and by the high compliance to radiotherapy. The grade 3/4 hematologic toxicities were more in NACT groups than CCRT (p < 0.001); the TPF group experienced more toxicity than PF (p = 0.029).
Conclusion: TPF/PF as NACT is feasible and produces impressive responses in cancer cervix.
Keywords: Cervical cancer; Disease-free survival; Hematological; Neoadjuvant chemotherapy.
Figures
References
-
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre): Summary report on HPV and cervical cancer statistics in India 2007. http://www.who.int/hpvcentre. Assessed 1 May 2008.
-
- FIGO staging for cancer cervix uteri . UICC manual for classification of malignant tumors. Berlin: Springer; 1987.
-
- Woo YJ, Byun JM, Jeong DH, et al. Prognosis of stage IIb cervical cancer among treatment regimens: radical hysterectomy versus neoadjuvant chemotherapy followed by radical hysterectomy versus concurrent chemoradiotherapy. Korean J Obstet Gynecol. 2012;55:913–919. doi: 10.5468/KJOG.2012.55.12.913. - DOI
-
- NCI Issues Clinical Announcement on Cervical Cancer, Chemotherapy Plus Radiation Improves Survival: http://www.nih.gov/news/pr/feb99/nci-22.htm.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous