Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: preparation optimization, in vitro dermal permeation, and in vivo bioevaluation
- PMID: 27486319
- PMCID: PMC4962759
- DOI: 10.2147/IJN.S105035
Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: preparation optimization, in vitro dermal permeation, and in vivo bioevaluation
Abstract
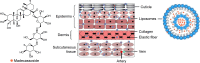
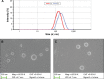
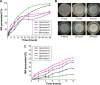
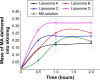
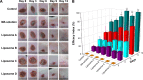
Madecassoside (MA) is highly potent in treating skin disorders such as wounds and psoriasis. However, the topical wound healing effect of MA was hampered by its poor membrane permeability. In order to overcome this shortcoming, MA liposomes were designed and prepared by a double-emulsion method to enhance transdermal and wound healing effects. In this study, response surface methodology was adopted to yield the optimal preparation conditions of MA double-emulsion liposomes with average particle size of 151 nm and encapsulation efficiency of 70.14%. Moreover, MA double-emulsion liposomes demonstrated superior stability and homogeneous appearance in 5 months; their leakage rate was <12% even at 37°C and <5% at 4°C within 1 month. In vitro skin permeation, skin distribution, and burn wound healing of MA liposomal formulations were conducted for the first time to evaluate MA delivery efficiency and wound healing effect. The transdermal property and wound cure effect of MA double-emulsion liposomes were superior to those of MA film dispersion liposomes, and both the methods were endowed with an excellent performance by polyethylene glycol modification. In conclusion, double-emulsion liposome formulation was an applicable and promising pharmaceutical preparation for enhancing MA delivery toward wound healing effect and improving wound-healing progress.
Keywords: double-emulsion method; madecassoside liposomes; skin permeation; transdermal property; wound-healing effect.
Figures


References
-
- Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of terminalia chebula on dermal wound healing in rats. Phytother Res. 2002;16(3):227–231. - PubMed
-
- Hardwicke J, Ferguson EL, Moseley R, Stephens P, Thomas DW, Duncan R. Dextrin-rhEGF conjugates as bioresponsive nanomedicines for wound repair. J Control Release. 2008;130(3):275–283. - PubMed
-
- Atiyeh BS, Hayek SN. An update on management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Med Chem Rev. 2004;1(2):111–121. - PubMed
-
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars. Am J Clin Dermatol. 2003;4(4):245–272. - PubMed
-
- Stella M, Castagnoli C, Gangemi EN. Postburn scars: an update. Int J Low Extrem Wounds. 2008;7(3):176–181. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

