Three CoA Transferases Involved in the Production of Short Chain Fatty Acids in Porphyromonas gingivalis
- PMID: 27486457
- PMCID: PMC4949257
- DOI: 10.3389/fmicb.2016.01146
Three CoA Transferases Involved in the Production of Short Chain Fatty Acids in Porphyromonas gingivalis
Abstract
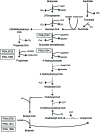
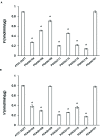
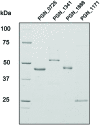
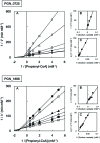
Butyryl-CoA:acetate CoA transferase, which produces butyrate and acetyl-CoA from butyryl-CoA and acetate, is responsible for the final step of butyrate production in bacteria. This study demonstrates that in the periodontopathogenic bacterium Porphyromonas gingivalis this reaction is not catalyzed by PGN_1171, previously annotated as butyryl-CoA:acetate CoA transferase, but by three distinct CoA transferases, PGN_0725, PGN_1341, and PGN_1888. Gas chromatography/mass spectrometry (GC-MS) and spectrophotometric analyses were performed using crude enzyme extracts from deletion mutant strains and purified recombinant proteins. The experiments revealed that, in the presence of acetate, PGN_0725 preferentially utilized butyryl-CoA rather than propionyl-CoA. By contrast, this preference was reversed in PGN_1888. The only butyryl-CoA:acetate CoA transferase activity was observed in PGN_1341. Double reciprocal plots revealed that all the reactions catalyzed by these enzymes follow a ternary-complex mechanism, in contrast to previously characterized CoA transferases. GC-MS analysis to determine the concentrations of short chain fatty acids (SCFAs) in culture supernatants of P. gingivalis wild type and mutant strains revealed that PGN_0725 and PGN_1888 play a major role in the production of butyrate and propionate, respectively. Interestingly, a triple deletion mutant lacking PGN_0725, PGN_1341, and PGN_1888 produced low levels of SCFAs, suggesting that the microorganism contains CoA transferase(s) in addition to these three enzymes. Growth rates of the mutant strains were mostly slower than that of the wild type, indicating that many carbon compounds produced in the SCFA synthesis appear to be important for the biological activity of this microorganism.
Keywords: CoA transferase; Porphyromonas gingivalis; butyrate; propionate; short chain fatty acid.
Figures



Similar articles
-
Acyl-CoA reductase PGN_0723 utilizes succinyl-CoA to generate succinate semialdehyde in a butyrate-producing pathway of Porphyromonas gingivalis.Arch Biochem Biophys. 2016 Apr 15;596:138-48. doi: 10.1016/j.abb.2016.03.014. Epub 2016 Mar 21. Arch Biochem Biophys. 2016. PMID: 27013206
-
Production of 4-hydroxybutyrate from succinate semialdehyde in butyrate biosynthesis in Porphyromonas gingivalis.Biochim Biophys Acta. 2015 Dec;1850(12):2582-91. doi: 10.1016/j.bbagen.2015.09.019. Biochim Biophys Acta. 2015. PMID: 26432601
-
Analysis of the Butyrate-Producing Pathway in Porphyromonas gingivalis.Methods Mol Biol. 2021;2210:167-172. doi: 10.1007/978-1-0716-0939-2_16. Methods Mol Biol. 2021. PMID: 32815137
-
Metabolic pathways for cytotoxic end product formation from glutamate- and aspartate-containing peptides by Porphyromonas gingivalis.J Bacteriol. 2000 Sep;182(17):4704-10. doi: 10.1128/JB.182.17.4704-4710.2000. J Bacteriol. 2000. PMID: 10940008 Free PMC article.
-
[Genes and gene clusters involved in microbial butyrate-producing pathway of human and animal gastrointestinal tract].Wei Sheng Wu Xue Bao. 2012 Oct 4;52(10):1181-6. Wei Sheng Wu Xue Bao. 2012. PMID: 23289315 Review. Chinese.
Cited by
-
Short-chain fatty acids-a key link between the gut microbiome and T-lymphocytes in neonates?Pediatr Res. 2025 Apr 30. doi: 10.1038/s41390-025-04075-0. Online ahead of print. Pediatr Res. 2025. PMID: 40307498 Review.
-
The concordance between upper and lower respiratory microbiota in children with Mycoplasma pneumoniae pneumonia.Emerg Microbes Infect. 2018 May 23;7(1):92. doi: 10.1038/s41426-018-0097-y. Emerg Microbes Infect. 2018. PMID: 29789582 Free PMC article.
-
Metabolic cooperativity between Porphyromonas gingivalis and Treponema denticola.J Oral Microbiol. 2020 Aug 24;12(1):1808750. doi: 10.1080/20002297.2020.1808750. J Oral Microbiol. 2020. PMID: 32944158 Free PMC article.
-
Butyryl/Caproyl-CoA:Acetate CoA-transferase: cloning, expression and characterization of the key enzyme involved in medium-chain fatty acid biosynthesis.Biosci Rep. 2021 Aug 27;41(8):BSR20211135. doi: 10.1042/BSR20211135. Biosci Rep. 2021. PMID: 34338280 Free PMC article.
-
Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion.Nat Commun. 2021 Sep 28;12(1):5674. doi: 10.1038/s41467-021-25965-x. Nat Commun. 2021. PMID: 34584098 Free PMC article.
References
-
- Duncan S. H., Barcenilla A., Stewart C. S., Pryde S. E., Flint H. J. (2002). Acetate utilization and butyryl coenzyme a (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 68 5186–5190. 10.1128/AEM.68.10.5186-5190.2002 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

