Post Hoc Analyses of Anxiety Measures in Adult Patients With Generalized Anxiety Disorder Treated With Vilazodone
- PMID: 27486544
- PMCID: PMC4956429
- DOI: 10.4088/PCC.15m01904
Post Hoc Analyses of Anxiety Measures in Adult Patients With Generalized Anxiety Disorder Treated With Vilazodone
Abstract
Objective: To investigate vilazodone, currently approved for major depressive disorder in adults, for generalized anxiety disorder (GAD).
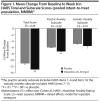
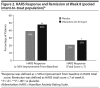
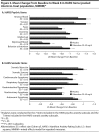
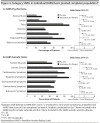
Method: Three randomized, double-blind, placebo-controlled studies showing positive results for vilazodone (2,040 mg/d) in adult patients with GAD (DSM-IV-TR) were pooled for analyses; data were collected from June 2012 to March 2014. Post hoc outcomes in the pooled intent-to-treat population (n = 1,462) included mean change from baseline to week 8 in Hamilton Anxiety Rating Scale (HARS) total score, psychic and somatic anxiety subscale scores, and individual item scores; HARS response (≥ 50% total score improvement) and remission (total score ≤ 7) at week 8; and category shifts, defined as HARS item score ≥ 2 at baseline (moderate to very severe symptoms) and score of 0 at week 8 (no symptoms).
Results: The least squares mean difference was statistically significant for vilazodone versus placebo in change from baseline to week 8 in HARS total score (-1.83, P < .0001) and in psychic anxiety (-1.21, P < .0001) and somatic anxiety (-0.63, P < .01) subscale scores; differences from placebo were significant on 11 of 14 HARS items (P < .05). Response rates were higher with vilazodone than placebo (48% vs 39%, P < .001), as were remission rates (27% vs 21%, P < .01). The percentage of patients who shifted to no symptoms was significant for vilazodone on several items: anxious mood, tension, intellectual, depressed mood, somatic-muscular, somatic-sensory, cardiovascular, respiratory, and autonomic symptoms (P < .05).
Conclusions: Treatment with vilazodone versus placebo was effective in adult GAD patients, with significant differences between treatment groups found on both psychic and somatic HARS items.
Trial registration: ClinicalTrials.gov identifiers: NCT01629966, NCT01766401, NCT01844115.
Similar articles
-
Efficacy and Safety of Vilazodone in Patients With Generalized Anxiety Disorder: A Randomized, Double-Blind, Placebo-Controlled, Flexible-Dose Trial.J Clin Psychiatry. 2016 Dec;77(12):1687-1694. doi: 10.4088/JCP.15m09885. J Clin Psychiatry. 2016. PMID: 27232052 Clinical Trial.
-
Vilazodone in patients with generalized anxiety disorder: a double-blind, randomized, placebo-controlled, flexible-dose study.Int Clin Psychopharmacol. 2015 Nov;30(6):297-306. doi: 10.1097/YIC.0000000000000096. Int Clin Psychopharmacol. 2015. PMID: 26291335 Free PMC article. Clinical Trial.
-
A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder.Depress Anxiety. 2015 Jun;32(6):451-9. doi: 10.1002/da.22365. Epub 2015 Apr 17. Depress Anxiety. 2015. PMID: 25891440 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of vilazodone for the acute treatment of generalized anxiety disorder: A meta-analysis.Asian J Psychiatr. 2017 Apr;26:115-122. doi: 10.1016/j.ajp.2017.01.016. Epub 2017 Jan 27. Asian J Psychiatr. 2017. PMID: 28483071
-
Vilazodone: a review in major depressive disorder in adults.Drugs. 2015 Nov;75(16):1915-23. doi: 10.1007/s40265-015-0490-y. Drugs. 2015. PMID: 26496736 Review.
References
-
- Fricchione G. Clinical practice. generalized anxiety disorder. N Engl J Med. 2004;351(7):675–682. - PubMed
-
- Mendlowicz MV, Stein MB. Quality of life in indivssiduals with anxiety disorders. Am J Psychiatry. 2000;157(5):669–682. - PubMed
-
- Bandelow B. Defining response and remission in anxiety disorders: toward an integrated approach. CNS Spectr. 2006;11(suppl 12):21–28. - PubMed
-
- Scholten WD, Batelaan NM, van Balkom AJ, et al. Recurrence of anxiety disorders and its predictors. J Affect Disord. 2013;147(1–3):180–185. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical