Efficacy and Safety of Human Retinal Progenitor Cells
- PMID: 27486556
- PMCID: PMC4959814
- DOI: 10.1167/tvst.5.4.6
Efficacy and Safety of Human Retinal Progenitor Cells
Erratum in
-
Erratum.Transl Vis Sci Technol. 2016 Dec 1;5(6):8. doi: 10.1167/tvst.5.6.8. eCollection 2016 Dec. Transl Vis Sci Technol. 2016. PMID: 27933218 Free PMC article.
Abstract
Purpose: We assessed the long-term efficacy and safety of human retinal progenitor cells (hRPC) using established rodent models.
Methods: Efficacy of hRPC was tested initially in Royal College of Surgeons (RCS) dystrophic rats immunosuppressed with cyclosporine/dexamethasone. Due to adverse effects of dexamethasone, this drug was omitted from a subsequent dose-ranging study, where different hRPC doses were tested for their ability to preserve visual function (measured by optokinetic head tracking) and retinal structure in RCS rats at 3 to 6 months after grafting. Safety of hRPC was assessed by subretinal transplantation into wild type (WT) rats and NIH-III nude mice, with analysis at 3 to 6 and 9 months after grafting, respectively.
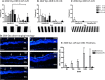
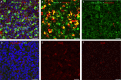
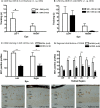
Results: The optimal dose of hRPC for preserving visual function/retinal structure in dystrophic rats was 50,000 to 100,000 cells. Human retinal progenitor cells integrated/survived in dystrophic and WT rat retina up to 6 months after grafting and expressed nestin, vimentin, GFAP, and βIII tubulin. Vision and retinal structure remained normal in WT rats injected with hRPC and there was no evidence of tumors. A comparison between dexamethasone-treated and untreated dystrophic rats at 3 months after grafting revealed an unexpected reduction in the baseline visual acuity of dexamethasone-treated animals.
Conclusions: Human retinal progenitor cells appear safe and efficacious in the preclinical models used here.
Translational relevance: Human retinal progenitor cells could be deployed during early stages of retinal degeneration or in regions of intact retina, without adverse effects on visual function. The ability of dexamethasone to reduce baseline visual acuity in RCS dystrophic rats has important implications for the interpretation of preclinical and clinical cell transplant studies.
Keywords: dexamethasone; nestin; retinal degeneration; retinal progenitor cells, human.
Figures




Similar articles
-
A Subsequent Human Neural Progenitor Transplant into the Degenerate Retina Does Not Compromise Initial Graft Survival or Therapeutic Efficacy.Transl Vis Sci Technol. 2015 Feb 10;4(1):7. doi: 10.1167/tvst.4.1.7. eCollection 2015 Feb. Transl Vis Sci Technol. 2015. PMID: 25694843 Free PMC article.
-
Human retinal progenitor cell transplantation preserves vision.J Biol Chem. 2014 Mar 7;289(10):6362-6371. doi: 10.1074/jbc.M113.513713. Epub 2014 Jan 9. J Biol Chem. 2014. PMID: 24407289 Free PMC article.
-
Gene expression changes in the retina following subretinal injection of human neural progenitor cells into a rodent model for retinal degeneration.Mol Vis. 2016 May 16;22:472-90. eCollection 2016. Mol Vis. 2016. PMID: 27217715 Free PMC article.
-
Human Retinal Progenitor Cells Derived Small Extracellular Vesicles Delay Retinal Degeneration: A Paradigm for Cell-free Therapy.Front Pharmacol. 2021 Nov 29;12:748956. doi: 10.3389/fphar.2021.748956. eCollection 2021. Front Pharmacol. 2021. PMID: 34912217 Free PMC article.
-
[Retinal pigment epithelial cell transplantation: perspective].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):982-1006. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022310 Review. Japanese.
Cited by
-
Intravitreal Injection of Human Retinal Progenitor Cells for Treatment of Retinal Degeneration.Med Sci Monit. 2020 Mar 28;26:e921184. doi: 10.12659/MSM.921184. Med Sci Monit. 2020. PMID: 32221273 Free PMC article.
-
Cellular regeneration strategies for macular degeneration: past, present and future.Eye (Lond). 2018 May;32(5):946-971. doi: 10.1038/s41433-018-0061-z. Epub 2018 Mar 5. Eye (Lond). 2018. PMID: 29503449 Free PMC article. Review.
-
Pluripotent Stem Cells for Retinal Tissue Engineering: Current Status and Future Prospects.Stem Cell Rev Rep. 2018 Aug;14(4):463-483. doi: 10.1007/s12015-018-9802-4. Stem Cell Rev Rep. 2018. PMID: 29675776 Free PMC article. Review.
-
Outer Retinal Cell Replacement: Putting the Pieces Together.Transl Vis Sci Technol. 2021 Aug 12;10(10):15. doi: 10.1167/tvst.10.10.15. Transl Vis Sci Technol. 2021. PMID: 34724034 Free PMC article. Review.
-
Techniques for subretinal injections in animals.Vet Ophthalmol. 2025 Mar;28(2):506-518. doi: 10.1111/vop.13219. Epub 2024 May 3. Vet Ophthalmol. 2025. PMID: 38700998 Free PMC article. Review.
References
-
- Marc RE,, Jones BW,, Watt CB,, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003; 22: 607–655. - PubMed
-
- Gal A,, Li Y,, Thompson DA,, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000; 26: 270–271. - PubMed
-
- Vugler AA. Progress toward the maintenance and repair of degenerating retinal circuitry. Retina. 2010; 30: 983–1001. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

