The lncRNA MALAT1 is a novel biomarker for gastric cancer metastasis
- PMID: 27486823
- PMCID: PMC5302908
- DOI: 10.18632/oncotarget.10941
The lncRNA MALAT1 is a novel biomarker for gastric cancer metastasis
Abstract
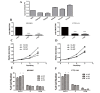
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is frequently over-expressed and serves as a prognostic marker in human cancers. However, little is known about the role of MALAT1 in gastric cancer. Here, we reported that the tissue and plasma MALAT1 levels were significantly higher in gastric cancer patients with distant metastasis (P<0.01) than patients without distant metastasis and the healthy controls. In addition, high levels of plasma MALAT1 independently correlated to a poor prognosis for gastric cancer patients (hazard ratio, 0.242; 95% CI, 0.154-0.836; P=0.036; Cox regression analysis). Functional studies revealed that knockdown of MALAT1 could inhibit cell proliferation, cell cycle progression, migration and invasion, and promote apoptosis in gastric cancer cells. Furthermore, the miR-122-IGF-1R signaling correlated with the dysregulated MALAT1 expression in gastric cancer. These data suggest that MALAT1 could function as an oncogene in gastric cancer, and high MALAT1 level could serve as a potential biomarker for the distant metastasis of gastric cancer.
Keywords: MALAT1; gastric cancer; long non-coding RNA; metastasis; miR-122.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

