Oncolytic virus therapy: A new era of cancer treatment at dawn
- PMID: 27486853
- PMCID: PMC5084676
- DOI: 10.1111/cas.13027
Oncolytic virus therapy: A new era of cancer treatment at dawn
Abstract
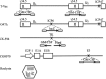
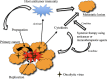
Oncolytic virus therapy is perhaps the next major breakthrough in cancer treatment following the success in immunotherapy using immune checkpoint inhibitors. Oncolytic viruses are defined as genetically engineered or naturally occurring viruses that selectively replicate in and kill cancer cells without harming the normal tissues. T-Vec (talimogene laherparepvec), a second-generation oncolytic herpes simplex virus type 1 (HSV-1) armed with GM-CSF, was recently approved as the first oncolytic virus drug in the USA and Europe. The phase III trial proved that local intralesional injections with T-Vec in advanced malignant melanoma patients can not only suppress the growth of injected tumors but also act systemically and prolong overall survival. Other oncolytic viruses that are closing in on drug approval in North America and Europe include vaccinia virus JX-594 (pexastimogene devacirepvec) for hepatocellular carcinoma, GM-CSF-expressing adenovirus CG0070 for bladder cancer, and Reolysin (pelareorep), a wild-type variant of reovirus, for head and neck cancer. In Japan, a phase II clinical trial of G47∆, a third-generation oncolytic HSV-1, is ongoing in glioblastoma patients. G47∆ was recently designated as a "Sakigake" breakthrough therapy drug in Japan. This new system by the Japanese government should provide G47∆ with priority reviews and a fast-track drug approval by the regulatory authorities. Whereas numerous oncolytic viruses have been subjected to clinical trials, the common feature that is expected to play a major role in prolonging the survival of cancer patients is an induction of specific antitumor immunity in the course of tumor-specific viral replication. It appears that it will not be long before oncolytic virus therapy becomes a standard therapeutic option for all cancer patients.
Keywords: Clinical trial; G47∆; herpes simplex virus; oncolytic immunotherapy; oncolytic virus.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 1956; 9: 1211–8. - PubMed
-
- Hoster H, Zanes R, von Haam E. The association of “viral” hepatitis and Hodgkin's disease. Cancer Res 1949; 9: 473–80. - PubMed
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther 2007; 15: 651–9. - PubMed
-
- Platanias LC. Mechanisms of type‐I‐ and type‐II‐interferon‐mediated signaling. Nat Rev Immunol 2005; 5: 375–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

