Host Nectin-1 Promotes Chlamydial Infection in the Female Mouse Genital Tract, but Is Not Required for Infection in a Novel Male Murine Rectal Infection Model
- PMID: 27486990
- PMCID: PMC4972247
- DOI: 10.1371/journal.pone.0160511
Host Nectin-1 Promotes Chlamydial Infection in the Female Mouse Genital Tract, but Is Not Required for Infection in a Novel Male Murine Rectal Infection Model
Abstract
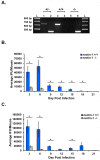
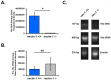
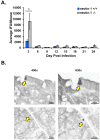
Chlamydia trachomatis is the most common bacterial sexually transmitted pathogen, but more than 70% of patients fail to seek treatment due to the asymptomatic nature of these infections. Women suffer from numerous complications from chronic chlamydial infections, which include pelvic inflammatory disease and infertility. We previously demonstrated in culture that host cell nectin-1 knockdown significantly reduced chlamydial titers and inclusion size. Here, we sought to determine whether nectin-1 was required for chlamydial development in vivo by intravaginally infecting nectin-1-/- mice with Chlamydia muridarum and monitoring chlamydial shedding by chlamydial titer assay. We observed a significant reduction in chlamydial shedding in female nectin-1-/- mice compared to nectin-1+/+ control mice, an observation that was confirmed by PCR. Immunohistochemical staining in mouse cervical tissue confirmed that there are fewer chlamydial inclusions in Chlamydia-infected nectin-1-/- mice. Notably, anorectal chlamydial infections are becoming a substantial health burden, though little is known regarding the pathogenesis of these infections. We therefore established a novel male murine model of rectal chlamydial infection, which we used to determine whether nectin-1 is required for anorectal chlamydial infection in male mice. In contrast to the data from vaginal infection, no difference in rectal chlamydial shedding was observed when male nectin-1+/+ and nectin-1-/- mice were compared. Through the use of these two models, we have demonstrated that nectin-1 promotes chlamydial infection in the female genital tract but does not appear to contribute to rectal infection in male mice. These models could be used to further characterize tissue and sex related differences in chlamydial infection.
Conflict of interest statement
Figures

References
-
- World Health Organization (2011) Global incidence and prevalence of selected curable sexually transmitted infections 2008. http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf. Accessed 24 Feb 2015
-
- Manavi K (2006) A review on infection with Chlamydia trachomatis. Best Pract Res Clin Obstet Gynaecol 20:941–51 - PubMed
-
- Paavonen J (2004) Sexually transmitted chlamydial infections and subfertility. Int Congr Ser 1266:277–286
-
- van Liere GAFS, Hoebe CJPA, Niekamp A-M, Koedijk FDH, Dukers-Muijrers NHTM (2013) Standard symptom- and sexual history-based testing misses anorectal Chlamydia trachomatis and neisseria gonorrhoeae infections in swingers and men who have sex with men. Sex Transm Dis 40:285–9 10.1097/OLQ.0b013e31828098f8 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

