Thermostability of Well-Ordered HIV Spikes Correlates with the Elicitation of Autologous Tier 2 Neutralizing Antibodies
- PMID: 27487086
- PMCID: PMC4972253
- DOI: 10.1371/journal.ppat.1005767
Thermostability of Well-Ordered HIV Spikes Correlates with the Elicitation of Autologous Tier 2 Neutralizing Antibodies
Abstract
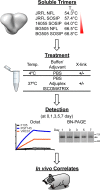
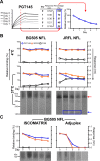
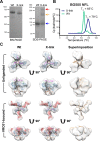
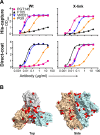
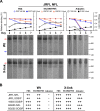
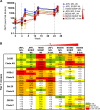
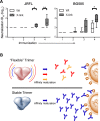
In the context of HIV vaccine design and development, HIV-1 spike mimetics displaying a range of stabilities were evaluated to determine whether more stable, well-ordered trimers would more efficiently elicit neutralizing antibodies. To begin, in vitro analysis of trimers derived from the cysteine-stabilized SOSIP platform or the uncleaved, covalently linked NFL platform were evaluated. These native-like trimers, derived from HIV subtypes A, B, and C, displayed a range of thermostabilities, and were "stress-tested" at varying temperatures as a prelude to in vivo immunogenicity. Analysis was performed both in the absence and in the presence of two different adjuvants. Since partial trimer degradation was detected at 37°C before or after formulation with adjuvant, we sought to remedy such an undesirable outcome. Cross-linking (fixing) of the well-ordered trimers with glutaraldehyde increased overall thermostability, maintenance of well-ordered trimer integrity without or with adjuvant, and increased resistance to solid phase-associated trimer unfolding. Immunization of unfixed and fixed well-ordered trimers into animals revealed that the elicited tier 2 autologous neutralizing activity correlated with overall trimer thermostability, or melting temperature (Tm). Glutaraldehyde fixation also led to higher tier 2 autologous neutralization titers. These results link retention of trimer quaternary packing with elicitation of tier 2 autologous neutralizing activity, providing important insights for HIV-1 vaccine design.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–82. . - PubMed
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–8. . - PubMed
-
- Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, et al. A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9(9):e1003618 10.1371/journal.ppat.1003618 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

