Mechanism of arginine sensing by CASTOR1 upstream of mTORC1
- PMID: 27487210
- PMCID: PMC4988899
- DOI: 10.1038/nature19079
Mechanism of arginine sensing by CASTOR1 upstream of mTORC1
Abstract
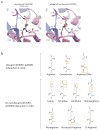
The mechanistic Target of Rapamycin Complex 1 (mTORC1) is a major regulator of eukaryotic growth that coordinates anabolic and catabolic cellular processes with inputs such as growth factors and nutrients, including amino acids. In mammals arginine is particularly important, promoting diverse physiological effects such as immune cell activation, insulin secretion, and muscle growth, largely mediated through activation of mTORC1 (refs 4, 5, 6, 7). Arginine activates mTORC1 upstream of the Rag family of GTPases, through either the lysosomal amino acid transporter SLC38A9 or the GATOR2-interacting Cellular Arginine Sensor for mTORC1 (CASTOR1). However, the mechanism by which the mTORC1 pathway detects and transmits this arginine signal has been elusive. Here, we present the 1.8 Å crystal structure of arginine-bound CASTOR1. Homodimeric CASTOR1 binds arginine at the interface of two Aspartate kinase, Chorismate mutase, TyrA (ACT) domains, enabling allosteric control of the adjacent GATOR2-binding site to trigger dissociation from GATOR2 and downstream activation of mTORC1. Our data reveal that CASTOR1 shares substantial structural homology with the lysine-binding regulatory domain of prokaryotic aspartate kinases, suggesting that the mTORC1 pathway exploited an ancient, amino-acid-dependent allosteric mechanism to acquire arginine sensitivity. Together, these results establish a structural basis for arginine sensing by the mTORC1 pathway and provide insights into the evolution of a mammalian nutrient sensor.
Figures

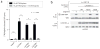







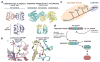
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

