Asymmetric division of contractile domains couples cell positioning and fate specification
- PMID: 27487217
- PMCID: PMC4998956
- DOI: 10.1038/nature18958
Asymmetric division of contractile domains couples cell positioning and fate specification
Abstract
During pre-implantation development, the mammalian embryo self-organizes into the blastocyst, which consists of an epithelial layer encapsulating the inner-cell mass (ICM) giving rise to all embryonic tissues. In mice, oriented cell division, apicobasal polarity and actomyosin contractility are thought to contribute to the formation of the ICM. However, how these processes work together remains unclear. Here we show that asymmetric segregation of the apical domain generates blastomeres with different contractilities, which triggers their sorting into inner and outer positions. Three-dimensional physical modelling of embryo morphogenesis reveals that cells internalize only when differences in surface contractility exceed a predictable threshold. We validate this prediction using biophysical measurements, and successfully redirect cell sorting within the developing blastocyst using maternal myosin (Myh9)-knockout chimaeric embryos. Finally, we find that loss of contractility causes blastomeres to show ICM-like markers, regardless of their position. In particular, contractility controls Yap subcellular localization, raising the possibility that mechanosensing occurs during blastocyst lineage specification. We conclude that contractility couples the positioning and fate specification of blastomeres. We propose that this ensures the robust self-organization of blastomeres into the blastocyst, which confers remarkable regulative capacities to mammalian embryos.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





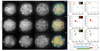

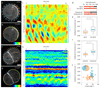
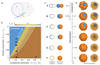
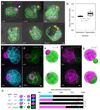
Comment in
-
Mammalian development: Mechanics drives cell differentiation.Nature. 2016 Aug 18;536(7616):281-2. doi: 10.1038/nature18920. Epub 2016 Aug 3. Nature. 2016. PMID: 27487214 No abstract available.
References
-
- Wennekamp S, Mesecke S, Nédélec F, Hiiragi T. A self-organization framework for symmetry breaking in the mammalian embryo. Nat Rev Mol Cell Biol. 2013;14:454–461. - PubMed
-
- Anani S, Bhat S, Honma-Yamanaka N, Krawchuk D, Yamanaka Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development. 2014;141:2813–2824. - PubMed
-
- Samarage CR, et al. Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. Dev Cell. 2015;34:435–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

