Medication Intervention for Chronic Kidney Disease Patients Transitioning from Hospital to Home: Study Design and Baseline Characteristics
- PMID: 27487357
- PMCID: PMC5155637
- DOI: 10.1159/000447019
Medication Intervention for Chronic Kidney Disease Patients Transitioning from Hospital to Home: Study Design and Baseline Characteristics
Abstract
Background: The hospital readmission rate in the population with chronic kidney disease (CKD) is high and strategies to reduce this risk are urgently needed.
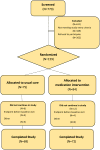
Methods: The CKD-Medication Intervention Trial (CKD-MIT; www.clinicaltrials.gov; NCTO1459770) is a single-blind (investigators), randomized, clinical trial conducted at Providence Health Care in Spokane, Washington. Study participants are hospitalized patients with CKD stages 3-5 (not treated with kidney replacement therapy) and acute illness. The study intervention is a pharmacist-led, home-based, medication management intervention delivered within 7 days after hospital discharge. The primary outcome is a composite of hospital readmissions and visits to emergency departments and urgent care centers for 90 days following hospital discharge. Secondary outcomes are achievements of guideline-based targets for CKD risk factors and complications.
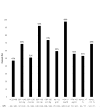
Results: Enrollment began in February 2012 and ended in May 2015. At baseline, the age of participants was 69 ± 11 years (mean ± SD), 50% (77 of 155) were women, 83% (117 of 141) had hypertension and 56% (79 of 141) had diabetes. At baseline, the estimated glomerular filtration rate was 41 ± 14 ml/min/1.73 m2 and urine albumin-to-creatinine ratio was 43 mg/g (interquartile range 8-528 mg/g). The most frequent diagnosis category for the index hospital admission was cardiovascular diseases at 34% (53 of 155), but the most common single diagnosis for admission was community-acquired acute kidney injury at 10% (16 of 155).
Conclusion: Participants in CKD-MIT are typical of acutely ill hospitalized patients with CKD. A medication management intervention after hospital discharge is under study to reduce post-hospitalization acute care utilization and to improve CKD management.
Trial registration: ClinicalTrials.gov NCT01459770.
© 2016 S. Karger AG, Basel.
Figures

References
-
- Daratha KB, Short RA, Corbett CF. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–416. - PubMed
-
- US RenalData System, USRDS Data Report: Atlas of chronic kidney disease in the United states. National Institute of Diabetes and Kidney Disease; Bethesda, MD: 2013.
-
- Hawes EM, Whitney DM, White SF. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transition. J Prim Care Community Health. 2014;5:14–18. - PubMed
-
- Forster AJ, Murff HJ, Peterson JF. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. - PubMed
-
- Doody HK, Peterson GM, Watson D. Retrospective evaluation of potentially inappropriate prescribing in hospitalized patients with renal impairment. Curr Med Res Opin. 2015;31:525–535. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

