Molecular Mechanisms of Innate Immune Inhibition by Non-Segmented Negative-Sense RNA Viruses
- PMID: 27487481
- PMCID: PMC5010489
- DOI: 10.1016/j.jmb.2016.07.017
Molecular Mechanisms of Innate Immune Inhibition by Non-Segmented Negative-Sense RNA Viruses
Abstract
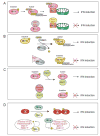
The host innate immune system serves as the first line of defense against viral infections. Germline-encoded pattern recognition receptors detect molecular patterns associated with pathogens and activate innate immune responses. Of particular relevance to viral infections are those pattern recognition receptors that activate type I interferon responses, which establish an antiviral state. The order Mononegavirales is composed of viruses that possess single-stranded, non-segmented negative-sense (NNS) RNA genomes and are important human pathogens that consistently antagonize signaling related to type I interferon responses. NNS viruses have limited encoding capacity compared to many DNA viruses, and as a likely consequence, most open reading frames encode multifunctional viral proteins that interact with host factors in order to evade host cell defenses while promoting viral replication. In this review, we will discuss the molecular mechanisms of innate immune evasion by select NNS viruses. A greater understanding of these interactions will be critical in facilitating the development of effective therapeutics and viral countermeasures.
Keywords: Mononegavirales; innate immune evasion; interferon antagonist; viral antagonism.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
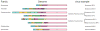


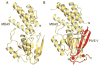

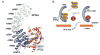

References
-
- Ortin J, Martin-Benito J. The RNA synthesis machinery of negative-stranded RNA viruses. Virology. 2015;479–480:532–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

