The use of digital PCR to improve the application of quantitative molecular diagnostic methods for tuberculosis
- PMID: 27487852
- PMCID: PMC4971652
- DOI: 10.1186/s12879-016-1696-7
The use of digital PCR to improve the application of quantitative molecular diagnostic methods for tuberculosis
Abstract
Background: Real-time PCR (qPCR) based methods, such as the Xpert MTB/RIF, are increasingly being used to diagnose tuberculosis (TB). While qualitative methods are adequate for diagnosis, the therapeutic monitoring of TB patients requires quantitative methods currently performed using smear microscopy. The potential use of quantitative molecular measurements for therapeutic monitoring has been investigated but findings have been variable and inconclusive. The lack of an adequate reference method and reference materials is a barrier to understanding the source of such disagreement. Digital PCR (dPCR) offers the potential for an accurate method for quantification of specific DNA sequences in reference materials which can be used to evaluate quantitative molecular methods for TB treatment monitoring.
Methods: To assess a novel approach for the development of quality assurance materials we used dPCR to quantify specific DNA sequences in a range of prototype reference materials and evaluated accuracy between different laboratories and instruments. The materials were then also used to evaluate the quantitative performance of qPCR and Xpert MTB/RIF in eight clinical testing laboratories.
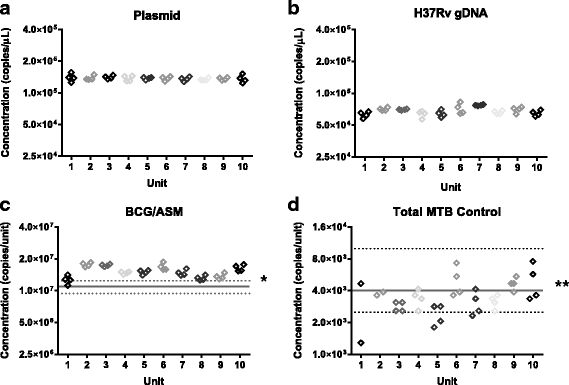
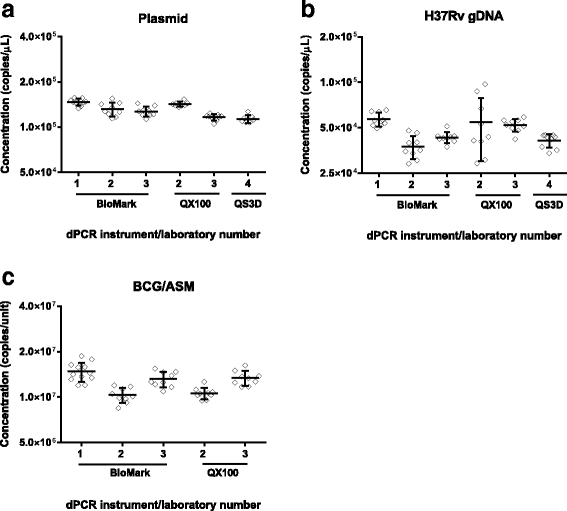
Results: dPCR was found to provide results in good agreement with the other methods tested and to be highly reproducible between laboratories without calibration even when using different instruments. When the reference materials were analysed with qPCR and Xpert MTB/RIF by clinical laboratories, all laboratories were able to correctly rank the reference materials according to concentration, however there was a marked difference in the measured magnitude.
Conclusions: TB is a disease where the quantification of the pathogen could lead to better patient management and qPCR methods offer the potential to rapidly perform such analysis. However, our findings suggest that when precisely characterised materials are used to evaluate qPCR methods, the measurement result variation is too high to determine whether molecular quantification of Mycobacterium tuberculosis would provide a clinically useful readout. The methods described in this study provide a means by which the technical performance of quantitative molecular methods can be evaluated independently of clinical variability to improve accuracy of measurement results. These will assist in ultimately increasing the likelihood that such approaches could be used to improve patient management of TB.
Keywords: Diagnostics; Digital PCR; Mycobacterium tuberculosis.
Figures

References
-
- World Health Organization: Policy Statement: Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva; 2011. - PubMed
-
- TB Xpert project - rolling out innovative MDR-TB Diagnostics [http://www.unitaid.eu/en/mdr-tb-diagnostics]. Accessed 27th Jan 2016.
-
- Public Health England: Position statement: Direct molecular testing for tuberculosis in England, Scotland and Wales; 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

