Development of bimetallic (Zn@Au) nanoparticles as potential PET-imageable radiosensitizers
- PMID: 27487895
- PMCID: PMC4967079
- DOI: 10.1118/1.4958961
Development of bimetallic (Zn@Au) nanoparticles as potential PET-imageable radiosensitizers
Abstract
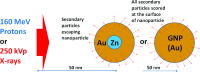
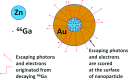
Purpose: Gold nanoparticles (GNPs) are being investigated actively for various applications in cancer diagnosis and therapy. As an effort to improve the imaging of GNPs in vivo, the authors developed bimetallic hybrid Zn@Au NPs with zinc cores and gold shells, aiming to render them in vivo visibility through positron emission tomography (PET) after the proton activation of the zinc core as well as capability to induce radiosensitization through the secondary electrons produced from the gold shell when irradiated by various radiation sources.
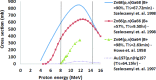
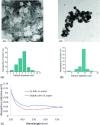
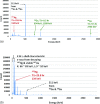
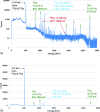
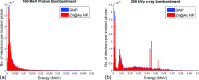
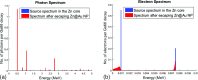
Methods: Nearly spherical zinc NPs (∼5-nm diameter) were synthesized and then coated with a ∼4.25-nm gold layer to make Zn@Au NPs (∼13.5-nm total diameter). 28.6 mg of these Zn@Au NPs was deposited (∼100 μm thick) on a thin cellulose target and placed in an aluminum target holder and subsequently irradiated with 14.15-MeV protons from a GE PETtrace cyclotron with 5-μA current for 5 min. After irradiation, the cellulose matrix with the NPs was placed in a dose calibrator to assess the induced radioactivity. The same procedure was repeated with 8-MeV protons. Gamma ray spectroscopy using an high-purity germanium detector was conducted on a very small fraction (<1 mg) of the irradiated NPs for each proton energy. In addition to experimental measurements, Monte Carlo simulations were also performed with radioactive Zn@Au NPs and solid GNPs of the same size irradiated with 160-MeV protons and 250-kVp x-rays.
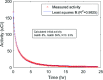
Results: The authors measured 168 μCi of activity 32 min after the end of bombardment for the 14.15-MeV proton energy sample using the (66)Ga setting on a dose calibrator; activity decreased to 2 μCi over a 24-h period. For the 8-MeV proton energy sample, PET imaging was additionally performed for 5 min after a 12-h delay. A 12-h gamma ray spectrum showed strong peaks at 511 keV (2.05 × 10(6) counts) with several other peaks of smaller magnitude for each proton energy sample. PET imaging showed strong PET signals from mostly decaying (66)Ga. The Monte Carlo results showed that radioactive Zn@Au NPs and solid GNPs provided similar characteristics in terms of their secondary electron spectra when irradiated.
Conclusions: The Zn@Au NPs developed in this investigation have the potential to be used as PET-imageable radiosensitizers for radiotherapy applications as well as PET tracers for molecular imaging applications.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

