Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss
- PMID: 27488235
- PMCID: PMC4997298
- DOI: 10.3945/ajcn.116.132563
Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss
Abstract
Background: A hypothesis exists whereby an exercise- or dietary-induced negative energy balance reduces human subcutaneous white adipose tissue (scWAT) mass through the formation of brown-like adipocyte (brite) cells. However, the validity of biomarkers of brite formation has not been robustly evaluated in humans, and clinical data that link brite formation and weight loss are sparse.
Objectives: We used rosiglitazone and primary adipocytes to stringently evaluate a set of biomarkers for brite formation and determined whether the expression of biomarker genes in scWAT could explain the change in body composition in response to exercise training combined with calorie restriction in obese and overweight women (n = 79).
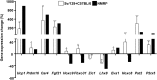
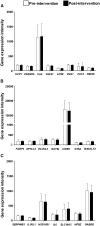
Design: Gene expression was derived from exon DNA microarrays and preadipocytes from obesity-resistant and -sensitive mice treated with rosiglitazone to generate candidate brite biomarkers from a microarray. These biomarkers were evaluated against data derived from scWAT RNA from obese and overweight women before and after supervised exercise 5 d/wk for 16 wk combined with modest calorie restriction (∼0.84 MJ/d).
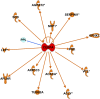
Results: Forty percent of commonly used brite gene biomarkers exhibited an exon or strain-specific regulation. No biomarkers were positively related to weight loss in human scWAT. Greater weight loss was significantly associated with less uncoupling protein 1 expression (P = 0.006, R(2) = 0.09). In a follow-up global analysis, there were 161 genes that covaried with weight loss that were linked to greater CCAAT/enhancer binding protein α activity (z = 2.0, P = 6.6 × 10(-7)), liver X receptor α/β agonism (z = 2.1, P = 2.8 × 10(-7)), and inhibition of leptin-like signaling (z = -2.6, P = 3.9 × 10(-5)).
Conclusion: We identify a subset of robust RNA biomarkers for brite formation and show that calorie-restriction-mediated weight loss in women dynamically remodels scWAT to take on a more-white rather than a more-brown adipocyte phenotype.
Keywords: browning; exercise; microarray; obesity; weight loss; white adipose tissue.
Figures



Comment in
-
Is human white adipose tissue bright during exercise?Am J Clin Nutr. 2016 Sep;104(3):545-6. doi: 10.3945/ajcn.116.141481. Epub 2016 Aug 17. Am J Clin Nutr. 2016. PMID: 27534635 No abstract available.
References
-
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–64. - PMC - PubMed
-
- Digby JE, Montague CT, Sewter CP, Sanders L, Wilkison WO, O’Rahilly S, Prins JB. Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 1998;47:138–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

