Anti-erythrocyte antibodies may contribute to anaemia in Plasmodium vivax malaria by decreasing red blood cell deformability and increasing erythrophagocytosis
- PMID: 27488382
- PMCID: PMC4973037
- DOI: 10.1186/s12936-016-1449-5
Anti-erythrocyte antibodies may contribute to anaemia in Plasmodium vivax malaria by decreasing red blood cell deformability and increasing erythrophagocytosis
Abstract
Background: Plasmodium vivax accounts for the majority of human malaria infections outside Africa and is being increasingly associated in fatal outcomes with anaemia as one of the major complications. One of the causes of malarial anaemia is the augmented removal of circulating non-infected red blood cells (nRBCs), an issue not yet fully understood. High levels of auto-antibodies against RBCs have been associated with severe anaemia and reduced survival of nRBCs in patients with falciparum malaria. Since there are no substantial data about the role of those antibodies in vivax malaria, this study was designed to determine whether or not auto-antibodies against erythrocytes are involved in nRBC clearance. Moreover, the possible immune mechanisms elicited by them that may be associated to induce anaemia in P. vivax infection was investigated.
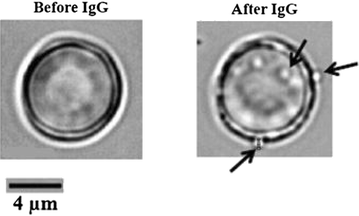
Methods: Concentrations of total IgG were determined by sandwich ELISA in sera from clinically well-defined groups of P. vivax-infected patients with or without anaemia and in healthy controls never exposed to malaria, whereas the levels of specific IgG to nRBCs were determined by cell-ELISA. Erythrophagocytosis assay was used to investigate the ability of IgGs purified from each studied pooled sera in enhancing nRBC in vitro clearance by THP-1 macrophages. Defocusing microscopy was employed to measure the biomechanical modifications of individual nRBCs opsonized by IgGs purified from each group.
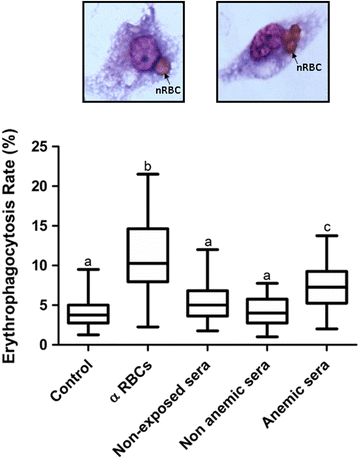
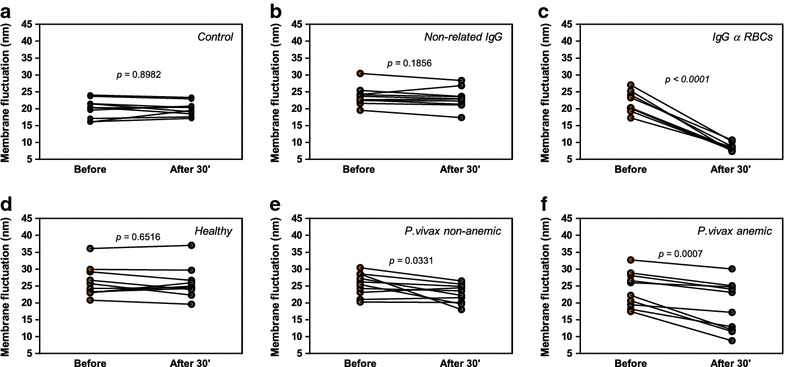
Results: Anaemic patients had higher levels of total and specific anti-RBC antibodies in comparison to the non-anaemic ones. Opsonization with purified IgG from anaemic patients significantly enhanced RBCs in vitro phagocytosis by THP-1 macrophages. Auto-antibodies purified from anaemic patients decreased the nRBC dynamic membrane fluctuations suggesting a possible participation of such antibodies in the perturbation of erythrocyte flexibility and morphology integrity maintenance.
Conclusions: These findings revealed that vivax-infected patients with anaemia have increased levels of IgG auto-antibodies against nRBCs and that their deposition on the surface of non-infected erythrocytes decreases their deformability, which, in turn, may enhance nRBC clearance by phagocytes, contributing to the anaemic outcome. These data provide insights into the immune mechanisms associated with vivax malaria anaemia and may be important to the development of new therapy and vaccine strategies.
Keywords: Anaemia; Auto-antibodies; Defocusing microscopy; Erythrophagocytosis; Non-infected RBC; Plasmodium vivax.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

