Listeria monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells
- PMID: 27488594
- PMCID: PMC5023727
- DOI: 10.1007/s00248-016-0809-6
Listeria monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells
Abstract
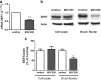
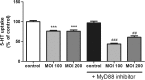
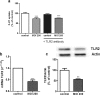
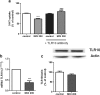
Listeria monocytogenes is a Gram-positive bacterium that can cause a serious infection. Intestinal microorganisms have been demonstrated to contribute to intestinal physiology not only through immunological responses but also by modulating the intestinal serotonergic system. Serotonin (5-HT) is a neuromodulator that is synthesized in the intestinal epithelium and regulates the whole intestinal physiology. The serotonin transporter (SERT), located in enterocytes, controls intestinal 5-HT availability and therefore serotonin's effects. Infections caused by L. monocytogenes are well described as being due to the invasion of intestinal epithelial cells; however, the effect of L. monocytogenes on the intestinal epithelium remains unknown. The main aim of this work, therefore, was to study the effect of L. monocytogenes on SERT. Caco2/TC7 cell line was used as an enterocyte-like in vitro model, and SERT functional and molecular expression assays were performed. Our results demonstrate that living L. monocytogenes inhibits serotonin uptake by reducing SERT expression at the brush border membrane. However, neither inactivated L. monocytogenes nor soluble metabolites were able to affect SERT. The results also demonstrate that L. monocytogenes yields TLR2 and TLR10 transcriptional changes in intestinal epithelial cells and suggest that TLR10 is potentially involved in the inhibitory effect observed on SERT. Therefore, L. monocytogenes, through TLR10-mediated SERT inhibition, may induce increased intestinal serotonin availability and potentially contributing to intestinal physiological changes and the initiation of the inflammatory response.
Keywords: 5-HT; Intestinal epithelium; Listeriosis; SERT; TLR.
Conflict of interest statement
Compliance with Ethical Standards Competing Interests The authors declare that they have no conflicts of interest.
Figures

References
-
- Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, Dittrich-Breiholz O, Kracht M, Hidmark A, Wigzell H, Rottenberg ME. Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner. Infect Immun. 2009;77(7):2908–2918. doi: 10.1128/IAI.01068-08. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

