Feature-Selective Attentional Modulations in Human Frontoparietal Cortex
- PMID: 27488638
- PMCID: PMC4971365
- DOI: 10.1523/JNEUROSCI.3935-15.2016
Feature-Selective Attentional Modulations in Human Frontoparietal Cortex
Abstract
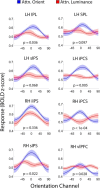
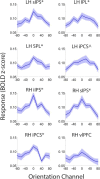
Control over visual selection has long been framed in terms of a dichotomy between "source" and "site," where top-down feedback signals originating in frontoparietal cortical areas modulate or bias sensory processing in posterior visual areas. This distinction is motivated in part by observations that frontoparietal cortical areas encode task-level variables (e.g., what stimulus is currently relevant or what motor outputs are appropriate), while posterior sensory areas encode continuous or analog feature representations. Here, we present evidence that challenges this distinction. We used fMRI, a roving searchlight analysis, and an inverted encoding model to examine representations of an elementary feature property (orientation) across the entire human cortical sheet while participants attended either the orientation or luminance of a peripheral grating. Orientation-selective representations were present in a multitude of visual, parietal, and prefrontal cortical areas, including portions of the medial occipital cortex, the lateral parietal cortex, and the superior precentral sulcus (thought to contain the human homolog of the macaque frontal eye fields). Additionally, representations in many-but not all-of these regions were stronger when participants were instructed to attend orientation relative to luminance. Collectively, these findings challenge models that posit a strict segregation between sources and sites of attentional control on the basis of representational properties by demonstrating that simple feature values are encoded by cortical regions throughout the visual processing hierarchy, and that representations in many of these areas are modulated by attention.
Significance statement: Influential models of visual attention posit a distinction between top-down control and bottom-up sensory processing networks. These models are motivated in part by demonstrations showing that frontoparietal cortical areas associated with top-down control represent abstract or categorical stimulus information, while visual areas encode parametric feature information. Here, we show that multivariate activity in human visual, parietal, and frontal cortical areas encode representations of a simple feature property (orientation). Moreover, representations in several (though not all) of these areas were modulated by feature-based attention in a similar fashion. These results provide an important challenge to models that posit dissociable top-down control and sensory processing networks on the basis of representational properties.
Keywords: frontoparietal cortex; functional neuroimaging; visual attention; visual cortex.
Copyright © 2016 the authors 0270-6474/16/368188-12$15.00/0.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
