A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors
- PMID: 27488640
- PMCID: PMC6601955
- DOI: 10.1523/JNEUROSCI.2783-15.2016
A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors
Abstract
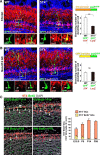
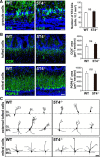
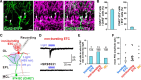
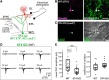
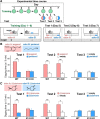
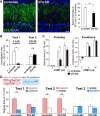
Neural circuits that undergo reorganization by newborn interneurons in the olfactory bulb (OB) are necessary for odor detection and discrimination, olfactory memory, and innate olfactory responses, including predator avoidance and sexual behaviors. The OB possesses many interneurons, including various types of granule cells (GCs); however, the contribution that each type of interneuron makes to olfactory behavioral control remains unknown. Here, we investigated the in vivo functional role of oncofetal trophoblast glycoprotein 5T4, a regulator for dendritic arborization of 5T4-expressing GCs (5T4 GCs), the level of which is reduced in the OB of 5T4 knock-out (KO) mice. Electrophysiological recordings with acute OB slices indicated that external tufted cells (ETCs) can be divided into two types, bursting and nonbursting. Optogenetic stimulation of 5T4 GCs revealed their connection to both bursting and nonbursting ETCs, as well as to mitral cells (MCs). Interestingly, nonbursting ETCs received fewer inhibitory inputs from GCs in 5T4 KO mice than from those in wild-type (WT) mice, whereas bursting ETCs and MCs received similar inputs in both mice. Furthermore, 5T4 GCs received significantly fewer excitatory inputs in 5T4 KO mice. Remarkably, in olfactory behavior tests, 5T4 KO mice had higher odor detection thresholds than the WT, as well as defects in odor discrimination learning. Therefore, the loss of 5T4 attenuates inhibitory inputs from 5T4 GCs to nonbursting ETCs and excitatory inputs to 5T4 GCs, contributing to disturbances in olfactory behavior. Our novel findings suggest that, among the various types of OB interneurons, the 5T4 GC subtype is required for odor detection and discrimination behaviors.
Significance statement: Neuronal circuits in the brain include glutamatergic principal neurons and GABAergic interneurons. Although the latter is a minority cell type, they are vital for normal brain function because they regulate the activity of principal neurons. If interneuron function is impaired, brain function may be damaged, leading to behavior disorder. The olfactory bulb (OB) possesses various types of interneurons, including granule cells (GCs); however, the contribution that each type of interneuron makes to the control of olfactory behavior remains unknown. Here, we analyzed electrophysiologically and behaviorally the function of oncofetal trophoblast glycoprotein 5T4, a regulator for dendritic branching in OB GCs. We found that, among the various types of OB interneuron, the 5T4 GC subtype is required for odor detection and odor discrimination behaviors.
Keywords: dendritic arborization; odor detection; odor discrimination; olfactory bulb interneuron; optogenetics; transmembrane protein.
Copyright © 2016 the authors 0270-6474/16/368211-18$15.00/0.
Figures




References
-
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An interglomerular circuit gates glomerular output and implements gain control in the mouse olfactory bulb. Neuron. 2015;87:193–207. doi: 10.1016/j.neuron.2015.06.019. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
