Load-induced enhancement of Dynein force production by LIS1-NudE in vivo and in vitro
- PMID: 27489054
- PMCID: PMC4976208
- DOI: 10.1038/ncomms12259
Load-induced enhancement of Dynein force production by LIS1-NudE in vivo and in vitro
Abstract
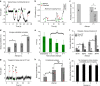
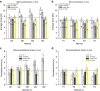
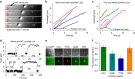
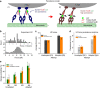
Most sub-cellular cargos are transported along microtubules by kinesin and dynein molecular motors, but how transport is regulated is not well understood. It is unknown whether local control is possible, for example, by changes in specific cargo-associated motor behaviour to react to impediments. Here we discover that microtubule-associated lipid droplets (LDs) in COS1 cells respond to an optical trap with a remarkable enhancement in sustained force production. This effect is observed only for microtubule minus-end-moving LDs. It is specifically blocked by RNAi for the cytoplasmic dynein regulators LIS1 and NudE/L (Nde1/Ndel1), but not for the dynactin p150(Glued) subunit. It can be completely replicated using cell-free preparations of purified LDs, where duration of LD force production is more than doubled. These results identify a novel, intrinsic, cargo-associated mechanism for dynein-mediated force adaptation, which should markedly improve the ability of motor-driven cargoes to overcome subcellular obstacles.
Figures


Comment in
-
Dynein: Let's not get stuck!Cell Cycle. 2017 Jan 2;16(1):7-8. doi: 10.1080/15384101.2016.1232085. Epub 2016 Sep 22. Cell Cycle. 2017. PMID: 27657557 Free PMC article. No abstract available.
References
-
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

