Neuroinvasion of α-Synuclein Prionoids after Intraperitoneal and Intraglossal Inoculation
- PMID: 27489279
- PMCID: PMC5044858
- DOI: 10.1128/JVI.01399-16
Neuroinvasion of α-Synuclein Prionoids after Intraperitoneal and Intraglossal Inoculation
Abstract
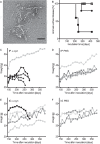
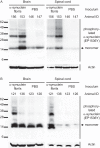
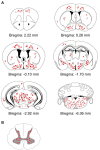
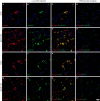
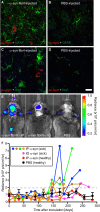
α-Synuclein is a soluble, cellular protein that in a number of neurodegenerative diseases, including Parkinson's disease and multiple system atrophy, forms pathological deposits of protein aggregates. Because misfolded α-synuclein has some characteristics that resemble those of prions, we investigated its potential to induce disease after intraperitoneal or intraglossal challenge injection into bigenic Tg(M83(+/-):Gfap-luc(+/-)) mice, which express the A53T mutant of human α-synuclein and firefly luciferase. After a single intraperitoneal injection with α-synuclein fibrils, four of five mice developed paralysis and α-synuclein pathology in the central nervous system, with a median incubation time of 229 ± 17 days. Diseased mice accumulated aggregates of Sarkosyl-insoluble and phosphorylated α-synuclein in the brain and spinal cord, which colocalized with ubiquitin and p62 and were accompanied by gliosis. In contrast, only one of five mice developed α-synuclein pathology in the central nervous system after intraglossal injection with α-synuclein fibrils, after 285 days. These findings are novel and important because they show that, similar to prions, α-synuclein prionoids can neuroinvade the central nervous system after intraperitoneal or intraglossal injection and can cause neuropathology and disease.
Importance: Synucleinopathies are neurodegenerative diseases that are characterized by the pathological presence of aggregated α-synuclein in cells of the nervous system. Previous studies have shown that α-synuclein aggregates made of recombinant protein or derived from brains of patients can spread in the central nervous system in a spatiotemporal manner when inoculated into the brains of animals and can induce pathology and neurologic disease, suggesting that misfolded α-synuclein can behave similarly to prions. Here we show that α-synuclein inoculation into the peritoneal cavity or the tongue in mice overexpressing α-synuclein causes neurodegeneration after neuroinvasion from the periphery, which further corroborates the prionoid character of misfolded α-synuclein.
Copyright © 2016 Breid et al.
Figures



References
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276:2045–2047. doi:10.1126/science.276.5321.2045. - DOI - PubMed
-
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. 2003. Alpha-synuclein locus triplication causes Parkinson's disease. Science 302:841. doi:10.1126/science.1090278. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

