Biotrophy at Its Best: Novel Findings and Unsolved Mysteries of the Arabidopsis-Powdery Mildew Pathosystem
- PMID: 27489521
- PMCID: PMC4957506
- DOI: 10.1199/tab.0184
Biotrophy at Its Best: Novel Findings and Unsolved Mysteries of the Arabidopsis-Powdery Mildew Pathosystem
Abstract
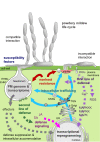
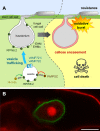
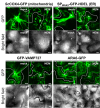
It is generally accepted in plant-microbe interactions research that disease is the exception rather than a common outcome of pathogen attack. However, in nature, plants with symptoms that signify colonization by obligate biotrophic powdery mildew fungi are omnipresent. The pervasiveness of the disease and the fact that many economically important plants are prone to infection by powdery mildew fungi drives research on this interaction. The competence of powdery mildew fungi to establish and maintain true biotrophic relationships renders the interaction a paramount example of a pathogenic plant-microbe biotrophy. However, molecular details underlying the interaction are in many respects still a mystery. Since its introduction in 1990, the Arabidopsis-powdery mildew pathosystem has become a popular model to study molecular processes governing powdery mildew infection. Due to the many advantages that the host Arabidopsis offers in terms of molecular and genetic tools this pathosystem has great capacity to answer some of the questions of how biotrophic pathogens overcome plant defense and establish a persistent interaction that nourishes the invader while in parallel maintaining viability of the plant host.
Figures






References
-
- Acevedo-Garcia J., Kusch S., and Panstruga R. ( . 2014). Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytol. 204: 273– 281. - PubMed
-
- Adam L., and Somerville S.C. ( . 1996). Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 9: 341– 356. - PubMed
-
- Adam L., Ellwood S., Wilson I., Saenz G., Xiao S., Oliver R.P., Turner J.G., and Somerville S. ( . 1999). Comparison of Erysiphe cichoracearum and E. cruciferarum and a Survey of 360 Arabidopsis thaliana Accessions for Resistance to These Two Powdery Mildew Pathogens. Mol. Plant Microbe Interact. 12: 1031– 1043. - PubMed
-
- An Q., Hückelhoven R., Kogel K.H., and van Bel A.J. ( . 2006). Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 8: 1009– 1019. - PubMed
-
- Andersson M.X., Kourtchenko O., Dangl J.L., Mackey D., and Ellerström M. ( . 2006). Phospholipase-dependent signalling during the AvrRpm1-and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J. 47: 947– 959. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
