EphA5 and EphA6: regulation of neuronal and spine morphology
- PMID: 27489614
- PMCID: PMC4971699
- DOI: 10.1186/s13578-016-0115-5
EphA5 and EphA6: regulation of neuronal and spine morphology
Abstract
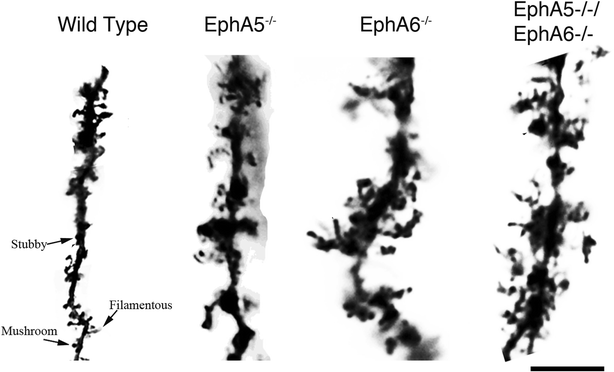
Background: The Eph family of receptor tyrosine kinases plays important roles in neural development. Previous studies have implicated Eph receptors and their ligands, the ephrins, in neuronal migration, axon bundling and guidance to specific targets, dendritic spine formation and neural plasticity. However, specific contributions of EphA5 and EphA6 receptors to the regulation of neuronal cell morphology have not been well studied.
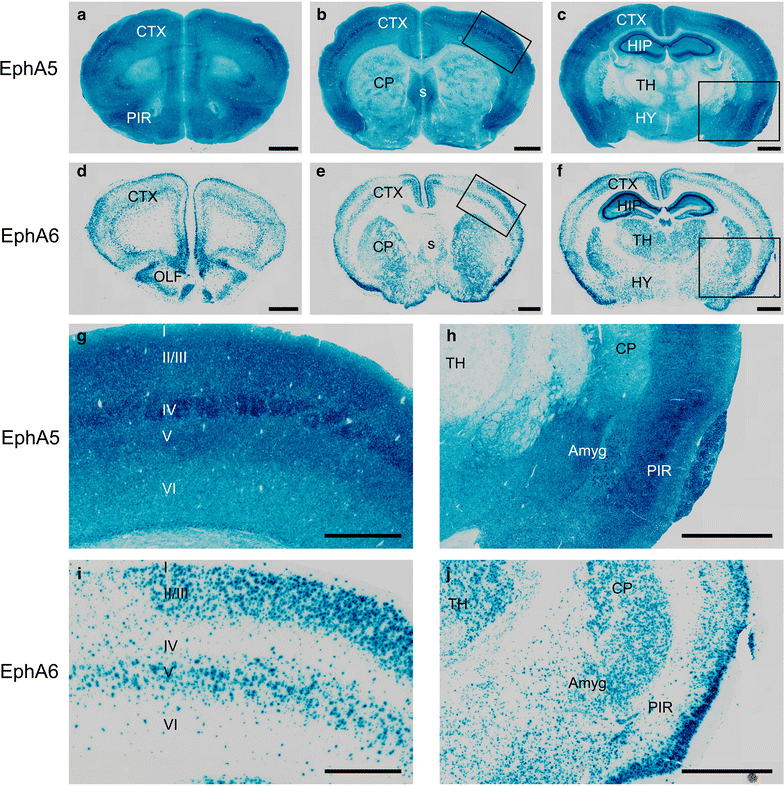
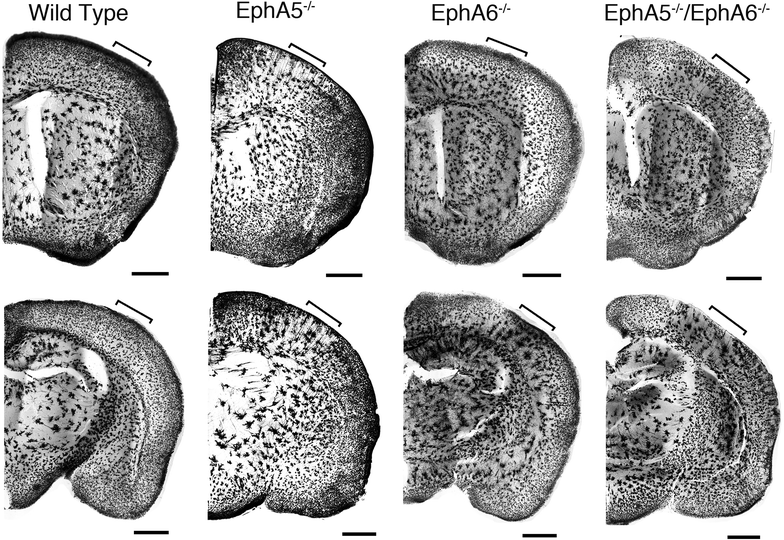
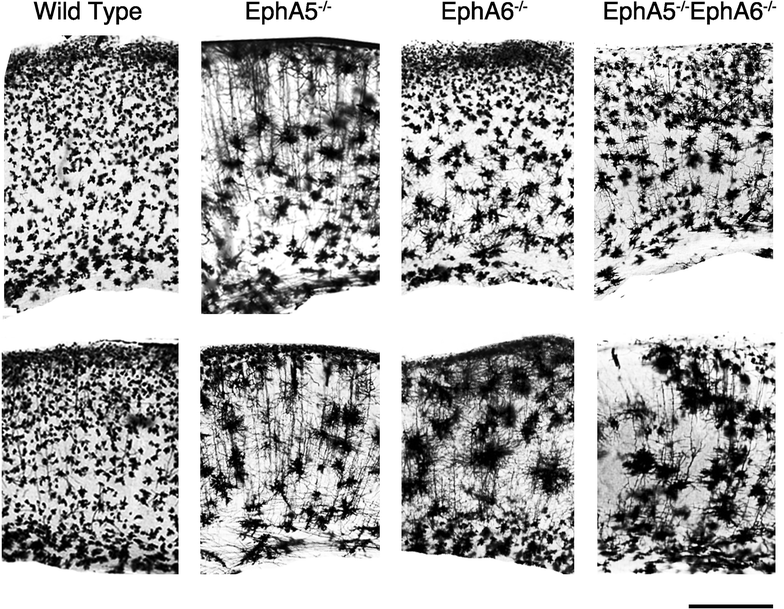
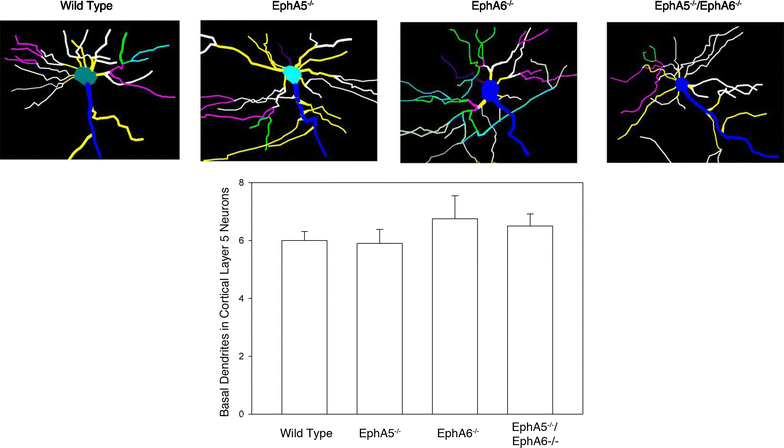
Results: Here we show that deletion of EphA5 and EphA6 results in abnormal Golgi staining patterns of cells in the brain, and abnormal spine morphology.
Conclusion: These observations suggest novel functions of these Eph receptors in the regulation of neuronal and spine structure in brain development and function.
Keywords: Cortex; Dendrite; EphA5; EphA6; Golgi staining; Spine.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

