The influence of a polymorphism in the gene encoding angiotensin converting enzyme (ACE) on treatment outcomes in late-onset Pompe patients receiving alglucosidase alfa
- PMID: 27489778
- PMCID: PMC4961277
- DOI: 10.1016/j.ymgmr.2016.07.005
The influence of a polymorphism in the gene encoding angiotensin converting enzyme (ACE) on treatment outcomes in late-onset Pompe patients receiving alglucosidase alfa
Abstract
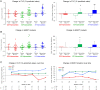
Correlations between angiotensin-converting enzyme (ACE) genotype (I/I, I/D, D/D), disease severity at baseline and response to enzyme replacement therapy (ERT) were assessed in the Pompe disease Late-Onset Treatment Study (LOTS). No correlations were observed between ACE genotype and disease severity at baseline. However, D/D patients appeared to have a reduced response to alglucosidase alfa treatment than I/I or I/D patients, suggesting that ACE polymorphisms may influence the response to alglucosidase alfa treatment and warrants further investigation.
Keywords: 6MWT, 6-Minute Walk Test; ACE, angiotensin-converting enzyme; Alglucosidase alfa; Angiotensin-converting enzyme; D, deletion; ERT, enzyme replacement therapy; Enzyme replacement therapy; FVC, forced vital capacity; GAA, acid-alpha glucosidase; Gene polymorphism; I, insertion; IOPD, infantile-onset Pompe disease; LOPD, late-onset Pompe disease; LOTS, Late-Onset Treatment Study; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; Pompe disease; QMT, quantitative muscle testing.
Figures
References
-
- van der Ploeg A.T., Reuser A.J. Pompe's disease. Lancet. 2008;372:1342–1353. - PubMed
-
- Hirschhorn R., Reuser A.J.J. Glycogen storage disease type II: acid α-glucosidase (acid maltase deficiency) In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Kinzler K.W., Vogelstein B., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001.
-
- Toscano A., Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J. Neurol. 2013;260:951–959. - PubMed
-
- van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L., Lange D.J., Leshner R.T., Mayhew J.E., Morgan C., Nozaki K., Park D.J., Pestronk A., Rosenbloom B., Skrinar A., van Capelle C.I., van der Beek N.A., Wasserstein M., Zivkovic S.A. A randomized study of alglucosidase alfa in late-onset Pompe's disease. New. Engl. J. Med. 2010;362:1396–1406. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

