Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs
- PMID: 27490003
- PMCID: PMC8504434
- DOI: 10.1002/14651858.CD011319.pub2
Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs
Abstract
Background: Atherosclerotic peripheral arterial disease (PAD) can lead to disabling ischemia and limb loss. Treatment modalities have included risk factor optimization through life-style modifications and medications, or operative approaches using both open and minimally invasive techniques, such as balloon angioplasty. Drug-eluting balloon (DEB) angioplasty has emerged as a promising alternative to uncoated balloon angioplasty for the treatment of this difficult disease process. By ballooning and coating the inside of atherosclerotic vessels with cytotoxic agents, such as paclitaxel, cellular mechanisms responsible for atherosclerosis and neointimal hyperplasia are inhibited and its devastating complications are prevented or postponed. DEBs are considerably more expensive than uncoated balloons, and their efficacy in improving patient outcomes is unclear.
Objectives: To assess the efficacy of drug-eluting balloons (DEBs) compared with uncoated, nonstenting balloon angioplasty in people with symptomatic lower-limb peripheral arterial disease (PAD).
Search methods: The Cochrane Vascular Trials Search Co-ordinator (TSC) searched the Specialised Register (last searched December 2015) and Cochrane Register of Studies (CRS) (2015, Issue 11). The TSC searched trial databases for details of ongoing and unpublished studies.
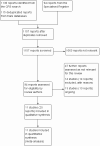
Selection criteria: We included all randomized controlled trials that compared DEBs with uncoated, nonstenting balloon angioplasty for intermittent claudication (IC) or critical limb ischemia (CLI).
Data collection and analysis: Two review authors (AK, TA) independently selected the appropriate trials and performed data extraction, assessment of trial quality, and data analysis. The senior review author (DKR) adjudicated any disagreements.
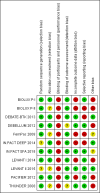
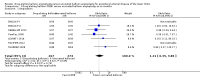
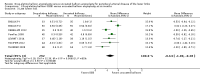
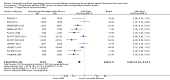
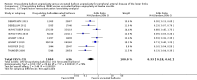
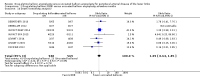
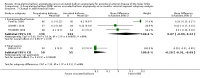
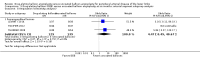
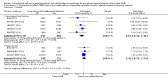
Main results: Eleven trials that randomized 1838 participants met the study inclusion criteria. Seven of the trials included femoropopliteal arterial lesions, three included tibial arterial lesions, and one included both. The trials were carried out in Europe and in the USA and all used the taxane drug paclitaxel in the DEB arm. Nine of the 11 trials were industry-sponsored. Four companies manufactured the DEB devices (Bard, Bavaria Medizin, Biotronik, and Medtronic). The trials examined both anatomic and clinical endpoints. There was heterogeneity in the frequency of stent deployment and the type and duration of antiplatelet therapy between trials. Using GRADE assessment criteria, the quality of the evidence presented was moderate for the outcomes of target lesion revascularization and change in Rutherford category, and high for amputation, primary vessel patency, binary restenosis, death, and change in ankle-brachial index (ABI). Most participants were followed up for 12 months, but one trial reported outcomes at five years.There were better outcomes for DEBs for up to two years in primary vessel patency (odds ratio (OR) 1.47, 95% confidence interval (CI) 0.22 to 9.57 at six months; OR 1.92, 95% CI 1.45 to 2.56 at 12 months; OR 3.51, 95% CI 2.26 to 5.46 at two years) and at six months and two years for late lumen loss (mean difference (MD) -0.64 mm, 95% CI -1.00 to -0.28 at six months; MD -0.80 mm, 95% CI -1.44 to -0.16 at two years). DEB were also superior to uncoated balloon angioplasty for up to five years in target lesion revascularization (OR 0.28, 95% CI 0.17 to 0.47 at six months; OR 0.40, 95% CI 0.31 to 0.51 at 12 months; OR 0.28, 95% CI 0.18 to 0.44 at two years; OR 0.21, 95% CI 0.09 to 0.51 at five years) and binary restenosis rate (OR 0.44, 95% CI 0.29 to 0.67 at six months; OR 0.38, 95% CI 0.15 to 0.98 at 12 months; OR 0.26, 95% CI 0.10 to 0.66 at two years; OR 0.12, 95% CI 0.05 to 0.30 at five years). There was no significant difference between DEB and uncoated angioplasty in amputation, death, change in ABI, change in Rutherford category and quality of life (QoL) scores, or functional walking ability, although none of the trials were powered to detect a significant difference in these clinical endpoints. We carried out two subgroup analyses to examine outcomes in femoropopliteal and tibial interventions as well as in people with CLI (4 or greater Rutherford class), and showed no advantage for DEBs in tibial vessels at six and 12 months compared with uncoated balloon angioplasty. There was also no advantage for DEBs in CLI compared with uncoated balloon angioplasty at 12 months.
Authors' conclusions: Based on a meta-analysis of 11 trials with 1838 participants, there is evidence of an advantage for DEBs compared with uncoated balloon angioplasty in several anatomic endpoints such as primary vessel patency (high-quality evidence), binary restenosis rate (moderate-quality evidence), and target lesion revascularization (low-quality evidence) for up to 12 months. Conversely, there is no evidence of an advantage for DEBs in clinical endpoints such as amputation, death, or change in ABI, or change in Rutherford category during 12 months' follow-up. Well-designed randomized trials with long-term follow-up are needed to compare DEBs with uncoated balloon angioplasties adequately for both anatomic and clinical study endpoints before the widespread use of this expensive technology can be justified.
Conflict of interest statement
AK: none known. TA: none known. GO: reports having received consultancy fees from Cordis Medical (provision of services for lectures around abdominal aortic aneurysm (AAA) and peripheral vascular disease (PVD) with no requirement to endorse Cordis products). GR: reports having received consultancy fees from Cordis Medical and Cook Medical related to giving lectures/proctoring on AAA and PVD with no exclusive relationship to promote their products. KT: reports that his institution is involved in many multicenter clinical trials and he is a principal investigator for: The IN.PACT Global Clinical Study for the Treatment of Comprehensive Superficial Femoral and/or Popliteal Artery Lesions using Drug‐Eluting Balloon; BIOFLEX‐I clinical study: The Treatment of Iliac and Femoral Atherosclerotic Lesions Using the Self‐expanding Astron and Astron Pulsar stents; Local Delivery of Paclitaxel for Prevention of Restenosis in Hemodialysis Access. Funds from Biotronic Inc and Medtronic Endovascular are managed independently by the department research board. KT declares payment for procuring and teaching of clinical specialists in advance aortic aneurysm stent graft (Cook Medical). Funds were also received by his institution for live course consultancy (Cordis), teaching and research activities from Cook Medical for the Advance EVAR [endovascular aneurysm repair] registry, Covidien grant for tumor ablation, and Gore Medical Teaching grants for fellows. These were also managed by the research department. DR: reports having received consultancy fees from TVA Medical (no commercially available products and the startup company (percutaneous creation of dialysis fistulas) has no interests within peripheral arterial disease); and Cordis Medical (provision of services for lectures around AAA and PVD with no requirement to endorse Cordis products).
Figures









































































Update of
- doi: 10.1002/14651858.CD011319
References
References to studies included in this review
BIOLUX P‐I {published and unpublished data}
-
- A prospective, multi‐centre, randomized controlled, first in man study to assess the safety and performance of the Passeo‐18 Lux paclitaxel releasing PTA balloon catheter vs. the uncoated Passeo 18 balloon catheter in patients with stenosis and occlusion of the femoropopliteal arteries (BIOLUX P‐I). clinicaltrials.gov/ct2/show/NCT01221610 (accessed 15 August 2015).
-
- Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel‐releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve‐month results from the BIOLUX P‐I randomized trial. Journal of Endovascular Therapy 2015;22:14‐21. - PubMed
-
- Tepe G, Schulte K‐L, Zeller T, Lammer J, Pfaffinger P, Schmidt A, et al. Six‐month results of the BIOLUX P‐I first in man study comparing a paclitaxel‐releasing balloon catheter versus an uncoated balloon catheter in femoropopliteal lesions. Cardiovascular and Interventional Radiology 2012; Vol. 35:S264‐5.
-
- Zeller T, Lammer J, Rastan A, Schulte K, Tepe G, Scheinert D. Twelve‐month results of the BIOLUX P‐I first‐in‐man study comparing a paclitaxel‐releasing balloon catheter versus an uncoated catheter in femoropopliteal lesions. Vasa ‐ Journal of Vascular Diseases 2013;42(Suppl 84):FV 9.7.
BIOLUX P‐II {published and unpublished data}
-
- BIOTONIK's ‐ first in men study of the Passeo‐18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo 18 balloon catheter in subjects requiring revascularization of infrapopliteal arteries (BIOLUX P‐II). clinicaltrials.gov/ct2/show/NCT01867736 (accessed 15 August 2015). - PubMed
-
- Deloose K, Zeller T, Scheinert D, Beschorner U. BIOLUX P‐II: a randomized clinical trial of Passeo‐18 Lux DRB vs POBA for the treatment of infrapopliteal artery lesions. Journal of the American College of Cardiology 2014; Vol. 64, issue Suppl B.
-
- Scheinert D, Brodmann M, Zeller T, Bosiers M, Peeters P, Karl‐Ludwig S, et al. BIOLUX P‐II: a randomized clinical trial of Passeo‐18 lux drug releasing balloon versus plain old balloon angioplasty for the treatment of infrapopliteal artery lesions. Journal of the American College of Cardiology 2014;64(11 Suppl 1):B79.
DEBATE‐BTK 2013 {published data only}
-
- Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, et al. Drug‐eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE‐BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013;128(6):615‐21. - PubMed
DEBELLUM 2012 {published and unpublished data}
-
- Fanelli F, Cannavale A, Boatta E, Corona M, Lucatelli P, Wlderk A, et al. Lower limb multilevel treatment with drug‐eluting balloons: 6‐month results from the DEBELLUM randomized trial. Journal of Endovascular Therapy 2012;19(5):571‐80. - PubMed
-
- Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The "DEBELLUM" ‐ lower limb multilevel treatment with drug eluting balloon ‐ randomized trial: 1‐year results. Journal of Cardiovascular Surgery 2014;55(2):207‐16. - PubMed
FemPac 2008 {published and unpublished data}
-
- Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel‐coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008;118(13):1358‐65. - PubMed
IN.PACT DEEP 2014 {published data only}
-
- Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, et al. Drug‐eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12‐month results from the IN.PACT DEEP randomized trial. Journal of the American College of Cardiology 2014;64(15):1568‐76. - PubMed
-
- Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, et al. IN.PACT Amphirion paclitaxel eluting balloon versus standard percutaneous transluminal angioplasty for infrapopliteal revascularization of critical limb ischemia: rationale and protocol for an ongoing randomized controlled trial. Trials 2014;15:63. - PMC - PubMed
IN.PACT SFA 2015 {published data only (unpublished sought but not used)}
-
- Laird JR, Schneider P, Tepe G, Brodmann M, Zeller T, Metzger C, et al. Durability of treatment effect using a drug‐coated balloon for femoropopliteal lesions 24‐month results of IN.PACT SFA. Journal of the American College of Cardiology 2015;66(21):2329‐38. - PubMed
-
- Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug‐coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12‐month results from the IN.PACT SFA randomized trial. Circulation 2015;131(5):495‐502. - PMC - PubMed
LEVANT I 2014 {published data only (unpublished sought but not used)}
-
- Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC. Cardiovascular Interventions 2014;7(1):10‐9. - PubMed
LEVANT II 2015 {published data only}
-
- Rosenfield K, Jaff MR, White CJ, Rocha‐Singh K, Mena‐Hurtado C, Metzger DC, et al. Trial of a paclitaxel‐coated balloon for femoropopliteal artery disease. New England Journal of Medicine 2015;373(2):145‐53. - PubMed
PACIFIER 2012 {published data only}
-
- Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, et al. Paclitaxel‐coated balloons reduce restenosis after femoro‐popliteal angioplasty: evidence from the randomized PACIFIER trial. Circulation. Cardiovascular Interventions 2012;5(6):831‐40. - PubMed
THUNDER 2008 {published data only}
-
- Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral‐popliteal arteries with drug‐coated balloons: 5‐year follow‐up of the THUNDER trial. JACC. Cardiovascular Interventions 2015;8(1):102‐8. - PubMed
-
- Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. New England Journal of Medicine 2008;358(7):689‐99. - PubMed
References to studies excluded from this review
COPA CABANA {published data only}
-
- Cotavance™ Paclitaxel‐coated balloon versus uncoated balloon angioplasty for treatment of in‐stent restenosis in SFA and popliteal arteries COPA CABANA Study. clinicaltrials.gov/ct2/show/NCT01594684 (accessed 15 August 2015).
DEBATE‐ISR {published data only}
-
- Liistro F, Angioli P, Porto I, Ricci L, Ducci K, Grotti S, et al. Paclitaxel‐eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in‐stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE‐ISR study. Journal of Endovascular Therapy 2014;21(1):1‐8. - PubMed
DEBATE SFA {published data only}
-
- Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, et al. Drug‐eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE‐SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC. Cardiovascular Interventions 2013;6(12):1295‐302. - PubMed
DEFINITIVE AR {published data only}
-
- Directional atherectomy followed by a paclitaxel‐coated balloon to inhibit restenosIs and maintain vessel patency: a pilot study of anti‐restenosis treatment. clinicaltrials.gov/ct2/show/NCT01366482 (accessed 15 August 2015).
EURO CANAL {published data only}
-
- European study of POBA versus Cotavance paclitaxel coated balloon for the treatment of infrapopliteal lesions in critical limb ischemia. clinicaltrials.gov/ct2/show/NCT01260870 (accessed 15 August 2015).
FAIR {published data only}
-
- Femoral artery in‐stent restenosis (FAIR) trial. clinicaltrials.gov/ct2/show/record/NCT01305070 (accessed 15 August 2015).
Freeway Stent Study {published data only}
-
- Tacke J, Kieselbach D, Schulte KL. [The Freeway stent study]. Presented at the Transcatheter Cardiovascular Therapeutics Conference in Washington, D.C. September 2014.
IDEAS {published data only}
-
- Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC. Cardiovascular Interventions 2014;7(9):1048‐56. - PubMed
ISAR‐PEBIS {published data only}
-
- Randomized trial of paclitaxel eluting balloon or conventional balloon for treatment of in‐stent restenosis of the superficial femoral artery in patients with symptomatic peripheral artery disease (ISAR‐PEBIS). clinicaltrials.gov/ct2/show/NCT01083394 (accessed 15 August 2015).
ISAR‐STATH {published data only}
-
- Randomized trial of stenting after dilation with or without paclitaxel eluting balloon or atherectomy in patients with symptomatic peripheral artery disease. clinicaltrials.gov/ct2/show/NCT00986752 (accessed 15 August 2015).
PACUBA 1 {published data only}
-
- A monocenter randomized clinical trial of PAClitaxel drUg‐eluting BAlloon versus standard percutaneous transluminal angioplasty to reduce restenosis in patients with in‐stent stenoses in the superficial femoral and proximal popliteal artery. clinicaltrials.gov/ct2/show/NCT01247402 (accessed 15 August 2015).
PHOTOPAC {published data only}
-
- Photoablative atherectomy followed by a paclitaxel‐coated balloon to inhibit restenosis in instent femoro‐popliteal obstructions. clinicaltrials.gov/ct2/show/NCT01298947 (accessed 15 August 2015). - PubMed
RAPID {published data only}
-
- Karimi A, Boer SW, Heuvel D, Fioole B, Vroegindeweij D, Heyligers JMM, et al. Randomized trial of Legflow® paclitaxel eluting balloon and stenting versus standard percutaneous transluminal angioplasty and stenting for the treatment of intermediate and long lesions of the superficial femoral artery (RAPID trial): study protocol for a randomized controlled trial. Trials 2013;14:87. - PMC - PubMed
SWEDEPAD {published data only}
-
- Swedish drug‐elution trial in peripheral arterial disease ‐ a multicenter, prospective randomized controlled clinical trial based on the Swedish Vascular Registry (SWEDVASC) Platform. clinicaltrials.gov/ct2/show/NCT02051088 (accessed 15 August 2015).
References to ongoing studies
ACOART‐BTK {published data only (unpublished sought but not used)}
-
- Evaluation of the use of ACOTEC drug‐eluting balloon Litos in below‐the‐knee arteries to treat critical limb ischemia (ACOART‐BTK). clinicaltrials.gov/ct2/show/NCT02563535 (accessed 25 December 2015).
AcoArt I {published data only (unpublished sought but not used)}
-
- Prospective, multi‐center and randomized controlled clinical study to verify effectiveness and safety of drug‐eluting balloon in PTA procedure (AcoArt I Study). clinicaltrials.gov/ct2/show/NCT01850056 (accessed 15 August 2015).
AcoArt II {published data only (unpublished sought but not used)}
-
- Prospective, multi‐center and randomized controlled clinical study to verify effectiveness and safety of drug‐eluting balloon in PTA procedure of the infrapopliteal artery. clinicaltrials.gov/ct2/show/NCT02137577 (accessed 15 August 2015).
Advance 18PTX Balloon Catheter Study {published data only (unpublished sought but not used)}
-
- Advance® 18PTX® Balloon Catheter Study: treatment of lesions in superficial femoral artery/popliteal artery with a paclitaxel‐coated balloon. clinicaltrials.gov/ct2/show/NCT00776906 (accessed 15 August 2015).
EFFPac {published data only (unpublished sought but not used)}
-
- Teichgräber U. Phase III multicenter randomized controlled trial to assess the effectiveness of paclitaxel‐coated Luminor balloon catheter versus uncoated balloon catheter in the superficial femoral and popliteal arteries to prevent vessel restenosis or reocclusion. Presented at the Leipzig Interventional Course; Leipzig, Germany January 2015.
FREERIDE Study {published data only (unpublished sought but not used)}
-
- Phase III FREERIDE study Freeway randomized angioplasty study. clinicaltrials.gov/ct2/show/NCT01960647 (accessed 15 August 2015).
ILLUMENATE {published data only (unpublished sought but not used)}
-
- Pivotal trial of a novel paclitaxel‐coated percutaneous angioplasty balloon. clinicaltrials.gov/ct2/show/NCT01858428 (accessed 20 December 2015).
LEVANT Japan {published data only (unpublished sought but not used)}
-
- A prospective, multicenter, single blind, randomized, controlled Japanese population trial comparing MD02‐LDCB versus standard balloon angioplasty for treatment of femoropopliteal arteries. clinicaltrials.gov/ct2/show/NCT01816412 (accessed 15 August 2015).
Lutonix BTK {published data only (unpublished sought but not used)}
-
- A prospective, multicenter, single blind, randomized, controlled trial comparing the Lutonix drug coated balloon versus standard balloon angioplasty for treatment of below‐the‐knee (BTK) arteries (Lutonix BTK trial). clinicaltrials.gov/ct2/show/NCT01870401 (accessed 15 August 2015).
MDT‐2113 SFA {published data only (unpublished sought but not used)}
-
- Randomized trial of MDT‐2113 drug‐eluting balloon (DEB) vs. standard PTA for the treatment of atherosclerotic lesions in the superficial femoral artery and/or proximal popliteal artery. clinicaltrials.gov/ct2/show/NCT01947478 (accessed 15 August 2015).
PICCOLO {published data only (unpublished sought but not used)}
-
- Paclitaxel coated balloons for prevention of restenosis in small arteries below the knee compared to angioplasty using uncoated balloons. clinicaltrials.gov/ct2/show/NCT00696956 (accessed 15 August 2015).
RANGER‐SFA {published data only (unpublished sought but not used)}
-
- Prospective, randomized, multicentre clinical study of the Hemoteq Ranger™ paclitaxel‐coated PTA balloon catheter (Ranger DCB) in comparison to uncoated PTA balloons in femoropopliteal lesions. clinicaltrials.gov/ct2/show/NCT02013193 (accessed 15 August 2015).
SINGA‐PACLI {published data only (unpublished sought but not used)}
-
- Singapore infra‐genicular angioplasty with paclitaxel‐eluting balloon for critical limb ischaemia (SINGA‐PACLI) trial. clinicaltrials.gov/ct2/show/NCT02129634 (accessed 15 August 2015).
Additional references
Aquino 2001
-
- Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, et al. Natural history of claudication: long‐term serial follow‐up study of 1244 claudicants. Journal of Vascular Surgery 2001;34(6):962‐70. - PubMed
Axel 1997
-
- Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997;96(2):636‐45. - PubMed
Baerlocher 2015
-
- Baerlocher MO, Kennedy SA, Rajebi MR, Baerlocher FJ, Misra J, Liu D, et al. Meta‐analysis of drug‐eluting balloon angioplasty and drug‐eluting stent placement for infrainguinal peripheral arterial disease. Journal of Vascular and Interventional Radiology 2015;26(4):459‐73. - PubMed
BASIL 2005
-
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366(9501):1925‐34. - PubMed
Bradbury 2010
-
- Bradbury AW, BASIL trial investigators and participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial in perspective. Journal of Vascular Surgery 2010;51(5 Suppl):1S‐4S. - PubMed
Canaud 2014
-
- Canaud L, Ozdemir BA, Belli AM, Loftus IM, Thompson MM, Hinchliffe RJ. Infrainguinal angioplasty with drug‐eluting stents and balloons. Journal of Vascular Surgery 2014;59:1721‐36. - PubMed
Cassese 2012
-
- Cassese S, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, et al. Paclitaxel‐coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta‐analysis of randomized trials. Circulation. Cardiovascular Interventions 2012;5:582‐9. - PubMed
Dake 2013
-
- Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Zilver PTX Investigators. Sustained safety and effectiveness of paclitaxel‐eluting stents for femoropopliteal lesions: 2‐year follow‐up from the Zilver PTX randomized and single‐arm clinical studies. Journal of the American College of Cardiology 2013;61(24):2417‐27. - PubMed
Fowkes 1991
-
- Fowkes FGR, Housley E, Cawood EHH, Macintyre CCA, Ruckley CV, Prescott RJ. Edinburgh artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384‐92. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 200
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463‐654. - PubMed
IDEAS 2014
-
- Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC. Cardiovascular Interventions 2014;7:1048‐56. - PubMed
Jens 2014a
-
- Jens S, Conijn AP, Koelemay MJW, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta‐analysis (part 1: above the knee). European Journal of Vascular and Endovascular Surgery 2014;47(5):524‐35. - PubMed
Jens 2014b
-
- Jens S, Conijn AP, Koelemay MJW, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta‐analysis (part 2: below the knee). European Journal of Vascular and Endovascular Surgery 2014;47(5):536‐44. - PubMed
Micari 2012
-
- Micari A, Cioppa A, Vadala G, Castriota F, Liso A, Marchese A, et al. Clinical evaluation of a paclitaxel‐eluting balloon for treatment of femoropopliteal arterial disease: 12‐month results from a multicenter Italian registry. JACC. Cardiovascular Interventions 2012;5:331‐8. - PubMed
Norgren 2007
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(Suppl S):S5‐67. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmidt 2011
-
- Schmidt A, Piorkowski M, Werner M, Ulrich M, Bausback Y, Braeunlich S, et al. First experience with drug‐eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. Journal of the American College of Cardiology 2011;58:1105‐9. - PubMed
Schnorr 2013
-
- Schnorr B, Albrecht T. Drug‐coated balloons and their place in treating peripheral arterial disease. Expert Review of Medical Devices 2013;10(1):105‐14. - PubMed
Seedial 2013
Selvin 2004
-
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation 2004;110(6):738‐43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

