Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes
- PMID: 27490266
- PMCID: PMC5032691
- DOI: 10.18632/aging.101007
Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes
Abstract
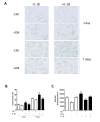
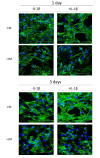
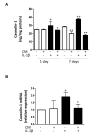
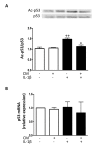
Aging and exposure to stress would determine the chondrocyte phenotype in osteoarthritis (OA). In particular, chronic inflammation may contribute to stress-induced senescence of chondrocytes and cartilage degeneration during OA progression. Recent studies have shown that adipose-derived mesenchymal stem cells exert paracrine effects protecting against degenerative changes in chondrocytes. We have investigated whether the conditioned medium (CM) from adipose-derived mesenchymal stem cells may regulate senescence features induced by inflammatory stress in OA chondrocytes. Our results indicate that CM down-regulated senescence markers induced by interleukin-1β including senescence-associated β-galactosidase activity, accumulation of γH2AX foci and morphological changes with enhanced formation of actin stress fibers. Treatment of chondrocytes with CM also decreased the production of oxidative stress, the activation of mitogen-activated protein kinases, and the expression of caveolin-1 and p21. The effects of CM were related to the reduction in p53 acetylation which would be dependent on the enhancement of Sirtuin 1 expression. Therefore, CM may exert protective effects in degenerative joint conditions by countering the premature senescence of OA chondrocytes induced by inflammatory stress.
Keywords: adipose-derived mesenchymal stem cells conditioned medium; chondrocytes; inflammation; senescence.
Conflict of interest statement
CONFLICT OF INTERESTS The authors declare no conflict of interests.
Figures



References
-
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26. - PubMed
-
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. - PubMed
-
- Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A:106–10. - PubMed
-
- Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

