Prevalence and Clinical Impact of Donor-Specific Alloantibody Among Intestinal Transplant Recipients
- PMID: 27490417
- PMCID: PMC7228620
- DOI: 10.1097/TP.0000000000001391
Prevalence and Clinical Impact of Donor-Specific Alloantibody Among Intestinal Transplant Recipients
Abstract
Background: Rejection remains the leading cause of allograft loss, and a major barrier to improving long-term outcomes after intestinal transplantation. Our aim is to define the prevalence and investigate the role of donor-specific antibody (DSA) on intestinal graft outcomes.
Methods: The study includes 109 transplants performed in 95 recipients at a single center. Patients were screened for DSA pretransplant, monitored regularly posttransplant and when clinically indicated using the single-antigen bead Luminex assay. Standard induction immunosuppression was with interleukin-2 receptor antagonists, and antithymocyte globulin in high-risk recipients. Maintenance regimens were tacrolimus-based.
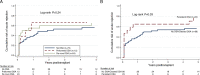
Results: Pretransplant DSA was detected in 12 (11%) recipients with 50% continuing to have circulating antibodies posttransplant. An additional 24 (25%) patients developed de novo DSA, and of these, 71% had persistent antibodies. Recipients with preformed DSA demonstrated elevated risks of early graft failure, whereas those with de novo DSA experienced accelerated graft loss once DSA was detected, reaching a 28% failure rate within 2 years. HLA-DQ mismatch is a significant risk factor for de novo DSA emergence, whereas the persistence of antibodies is predicted by DSA strength and specificity. Although inclusion of the liver in the intestinal allograft imparts an immunological advantage against rejection-related graft loss, this protective effect was lost among recipients with persistent DSA.
Conclusions: The presence of DSA is associated with inferior graft outcomes among intestinal transplant recipients. An enhanced understanding of the mechanisms by which DSA causes allograft injury, and effective strategies targeting humoral immune reactivity are needed to improve long-term intestinal graft outcomes.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures




References
-
- Terasaki PI. Humoral theory of transplantation Am J Transplant 2003. 3665–673 - PubMed
-
- Einecke G, Sis B, Reeve J. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure Am J Transplant 2009. 92520–2531 - PubMed
-
- Kaczmarek I, Deutsch MA, Kauke T. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant Exp Clin Transplant 2008. 6229–235 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
