High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors
- PMID: 27490492
- PMCID: PMC4973975
- DOI: 10.1371/journal.ppat.1005812
High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors
Abstract
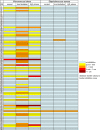
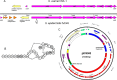
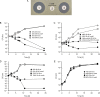
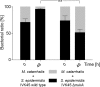
The human nasal microbiota is highly variable and dynamic often enclosing major pathogens such as Staphylococcus aureus. The potential roles of bacteriocins or other mechanisms allowing certain bacterial clones to prevail in this nutrient-poor habitat have hardly been studied. Of 89 nasal Staphylococcus isolates, unexpectedly, the vast majority (84%) was found to produce antimicrobial substances in particular under habitat-specific stress conditions, such as iron limitation or exposure to hydrogen peroxide. Activity spectra were generally narrow but highly variable with activities against certain nasal members of the Actinobacteria, Proteobacteria, Firmicutes, or several groups of bacteria. Staphylococcus species and many other Firmicutes were insusceptible to most of the compounds. A representative bacteriocin was identified as a nukacin-related peptide whose inactivation reduced the capacity of the producer Staphylococcus epidermidis IVK45 to limit growth of other nasal bacteria. Of note, the bacteriocin genes were found on mobile genetic elements exhibiting signs of extensive horizontal gene transfer and rearrangements. Thus, continuously evolving bacteriocins appear to govern bacterial competition in the human nose and specific bacteriocins may become important agents for eradication of notorious opportunistic pathogens from human microbiota.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Rosenthal M, Goldberg D, Aiello A, Larson E, Foxman B. Skin microbiota: microbial community structure and its potential association with health and disease. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11(5):839–48. Epub 2011/04/06. 10.1016/j.meegid.2011.03.022 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

