Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review
- PMID: 27490698
- PMCID: PMC4974007
- DOI: 10.1371/journal.pone.0157385
Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review
Abstract
Background: In the context of early vaccine trials aimed at evaluating the safety profile of novel vaccines, abnormal haematological values, such as neutropenia, are often reported. It is therefore important to evaluate how these trials should be planned not to miss potentially important safety signals, but also to understand the implications and the clinical relevance.
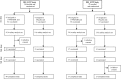
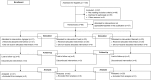
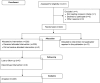
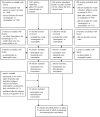
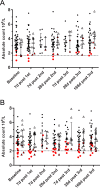
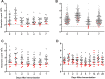
Methodology: We report and discuss the results from five clinical trials (two with a new Shigella vaccine in the early stage of clinical development and three with licensed vaccines) where the absolute neutrophil counts (ANC) were evaluated before and after vaccination. Additionally, we have performed a systematic review of the literature on cases of neutropenia reported during vaccine trials to discuss our results in a more general context.
Principal findings: Both in our clinical trials and in the literature review, several cases of neutropenia have been reported, in the first two weeks after vaccination. However, neutropenia was generally transient and had a benign clinical outcome, after vaccination with either multiple novel candidates or well-known licensed vaccines. Additionally, the vaccine recipients with neutropenia frequently had lower baseline ANC than non-neutropenic vaccinees. In many instances neutropenia occurred in subjects of African descent, known to have lower ANC compared to western populations.
Conclusions: It is important to include ANC and other haematological tests in early vaccine trials to identify potential safety signals. Post-vaccination neutropenia is not uncommon, generally transient and clinically benign, but many vaccine trials do not have a sampling schedule that allows its detection. Given ethnic variability in the level of circulating neutrophils, normal ranges taking into account ethnicity should be used for determination of trial inclusion/exclusion criteria and classification of neutropenia related adverse events.
Trial registration: ClinicalTrials.gov NCT02017899, NCT02034500, NCT01771367, NCT01765413, NCT02523287.
Conflict of interest statement
Figures
References
-
- Reading R. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Child: Care, Health and Development. 2008;34(4):548–. 10.1111/j.1365-2214.2008.00864_6.x - DOI
-
- Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Archives of internal medicine. 1991;151(3):501–5. Epub 1991/03/01. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

