Screening women for cervical cancer carcinoma with a HPV mRNA test: first results from the Venice pilot program
- PMID: 27490801
- PMCID: PMC4997543
- DOI: 10.1038/bjc.2016.216
Screening women for cervical cancer carcinoma with a HPV mRNA test: first results from the Venice pilot program
Abstract
Background: HPV DNA-based screening is more effective than a Pap test in preventing cervical cancer, but the test is less specific. New HPV tests have been proposed for primary screening. The HPV mRNA test showed a similar or slightly lower sensitivity than the HPV DNA tests but with a higher specificity. We report the results of an organised HPV mRNA-based screening pilot program in Venice, Italy.
Methods: From October 2011 to May 2014, women aged 25-64 years were invited to undergo a HPV mRNA test (Aptima). Those testing positive underwent cytological triage. Women with positive cytology were referred to colposcopy, whereas those with negative cytology were referred to repeat the HPV mRNA test 1 year later. The results of the HPV mRNA test program were compared with both the local historical cytology-based program and with four neighbouring DNA HPV-based pilot projects.
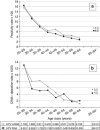
Results: Overall, 23 211 women underwent a HPV mRNA test. The age-standardised positivity rate was 7.0%, higher than in HPV DNA programs (6.8%; relative rate (RR) 1.11, 95% confidence interval (CI) 1.05-1.17). The total colposcopy referral was 5.1%, double than with cytology (2.6%; RR 2.02, 95% CI 1.82-2.25) but similar to the HPV DNA programs (4.8%; RR 1.02; 95% CI 0.96-1.08). The cervical intraepithelial neoplasia grade 2+ detection rate with HPV mRNA was greater than in the HPV DNA programs at baseline (RR 1.50; 95% CI 1.19-1.88) and not significantly lower at the 1-year repeat (RR 0.70; 95% CI 0.40-1.16). The overall RR was 1.29 (95% CI 1.05-1.59), which was much higher than with cytology (detection rate 5.5‰ vs 2.1‰; RR 2.50, 95% CI 1.76-3.62).
Conclusions: A screening programme based on the HPV mRNA obtained results similar to those observed with the HPV DNA test. In routine screening programmes, even a limited increase in HPV prevalence may conceal the advantage represented by the higher specificity of HPV mRNA.
Conflict of interest statement
Dr Paolo Giorgi Rossi, the Principal Investigator of an independent study funded by the Italian Ministry of Health, is negotiating with Roche Diagnostics, Hologic-Genprobe, Qiagen and Abbott to obtain reagents at a reduced price or free of charge. The remaining authors declare no conflict of interest.
Figures
References
-
- Arbyn M, Ronco G, Anttila A, CJLM Meijer, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J (2012) Evidence regarding HPV testing in secondary prevention of cervical cancer. Vaccine 30(Suppl 5): F88–F99. - PubMed
-
- Arbyn M, Haelens A, Desomer A, Verdoodt F, Thiry N, Francart J, Hanquet G, Robays J (2015. a) Cervical cancer screening program and Human Papillomavirus (HPV) testing, part II: update on HPV primary screening. Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE). KCE Reports 238: D/2015/10.273/17.
-
- Arbyn M, Snijders PJ, Meijer CJ, Berkhof J, Cuschieri K, Kocjan BJ, Poljak M (2015. b) Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect 21: 817–826. - PubMed
-
- Baussano I, Franceschi S, Gillio-Tos A, Carozzi F, Confortini M, Dalla Palma P, De Lillo M, Del Mistro A, De Marco L, Naldoni C, Pierotti P, Schincaglia P, Segnan N, Zorzi M, Giorgi-Rossi P, Ronco G (2013) Difference in overall and age-specific prevalence of high-risk human papillomavirus infection in Italy: evidence from NTCC trial. BMC Infect Dis 13: 238. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

