Deletion of FoxO1, 3, and 4 in Osteoblast Progenitors Attenuates the Loss of Cancellous Bone Mass in a Mouse Model of Type 1 Diabetes
- PMID: 27491024
- PMCID: PMC5492385
- DOI: 10.1002/jbmr.2934
Deletion of FoxO1, 3, and 4 in Osteoblast Progenitors Attenuates the Loss of Cancellous Bone Mass in a Mouse Model of Type 1 Diabetes
Abstract
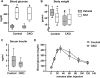
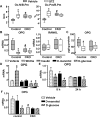
Type 1 diabetes is associated with osteopenia and increased fragility fractures, attributed to reduced bone formation. However, the molecular mechanisms mediating these effects remain unknown. Insulin promotes osteoblast formation and inhibits the activity of the FoxO transcription factors. FoxOs, on the other hand, inhibit osteoprogenitor proliferation and bone formation. Here, we investigated whether FoxOs play a role in the low bone mass associated with type 1 diabetes, using mice lacking FoxO1, 3, and 4 in osteoprogenitor cells (FoxO1,3,4ΔOsx1-Cre ). Streptozotocin-induced diabetes caused a reduction in bone mass and strength in FoxO-intact mice. In contrast, cancellous bone was unaffected in diabetic FoxO1,3,4ΔOsx1-Cre mice. The low bone mass in the FoxO-intact diabetic mice was associated with decreased osteoblast number and bone formation, as well as decreased expression of the anti-osteoclastogenic cytokine osteoprotegerin (OPG) and increased osteoclast number. FoxO deficiency did not alter the effects of diabetes on bone formation; however, it did prevent the decrease in OPG and the increase in osteoclast number. Addition of high glucose to osteoblastic cell cultures decreased OPG mRNA, indicating that hyperglycemia in and of itself contributes to diabetic bone loss. Taken together, these results suggest that FoxOs exacerbate the loss of cancellous bone mass associated with type 1 diabetes and that inactivation of FoxOs might ameliorate the adverse effects of insulin deficiency. © 2016 American Society for Bone and Mineral Research.
Keywords: BONE HISTOMORPHOMETRY; DISEASES AND DISORDERS OF/RELATED TO BONE; GENETIC ANIMAL MODELS; STROMAL/STEM CELLS; TRANSCRIPTION FACTORS.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures



References
-
- Compston JE, Smith EM, Matthews C, Schofield P. Whole body composition and regional bone mass in women with insulin-dependent diabetes mellitus. Clin Endocrinol (Oxf) 1994;41(3):289–93. - PubMed
-
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427–44. - PubMed
-
- Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone, sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8(5):297–305. - PubMed
-
- Hui SL, Epstein S, Johnston CC., Jr A prospective study of bone mass in patients with type I diabetes. J Clin Endocrinol Metab. 1985;60(1):74–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

