Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity
- PMID: 27491084
- PMCID: PMC5007983
- DOI: 10.1242/dmm.024703
Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity
Abstract
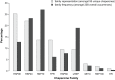
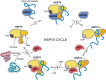
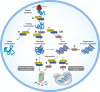
Chaperones and co-chaperones enable protein folding and degradation, safeguarding the proteome against proteotoxic stress. Chaperones display dynamic responses to exogenous and endogenous stressors and thus constitute a key component of the proteostasis network (PN), an intricately regulated network of quality control and repair pathways that cooperate to maintain cellular proteostasis. It has been hypothesized that aging leads to chronic stress on the proteome and that this could underlie many age-associated diseases such as neurodegeneration. Understanding the dynamics of chaperone function during aging and disease-related proteotoxic stress could reveal specific chaperone systems that fail to respond to protein misfolding. Through the use of suppressor and enhancer screens, key chaperones crucial for proteostasis maintenance have been identified in model organisms that express misfolded disease-related proteins. This review provides a literature-based analysis of these genetic studies and highlights prominent chaperone modifiers of proteotoxicity, which include the HSP70-HSP40 machine and small HSPs. Taken together, these studies in model systems can inform strategies for therapeutic regulation of chaperone functionality, to manage aging-related proteotoxic stress and to delay the onset of neurodegenerative diseases.
Keywords: Chaperome; Chaperone; Co-chaperone; Disease models; Protein-misfolding disease; Proteostasis network.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
-
- Al-Ramahi I., Lam Y. C., Chen H. K., De Gouyon B., Zhang M., Perez A. M., Branco J., De Haro M., Patterson C., Zoghbi H. Y., et al. (2006). CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 281, 26714-26724. 10.1074/jbc.M601603200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

