Reproductive age-associated fibrosis in the stroma of the mammalian ovary
- PMID: 27491879
- PMCID: PMC4979755
- DOI: 10.1530/REP-16-0129
Reproductive age-associated fibrosis in the stroma of the mammalian ovary
Abstract
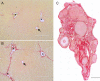
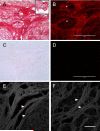
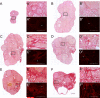
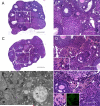
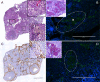
Under normal physiological conditions, tissue remodeling in response to injury leads to tissue regeneration without permanent damage. However, if homeostasis between synthesis and degradation of extracellular matrix (ECM) components is altered, fibrosis - or the excess accumulation of ECM - can disrupt tissue architecture and function. Several organs, including the heart, lung and kidney, exhibit age-associated fibrosis. Here we investigated whether fibrosis underlies aging in the ovary - an organ that ages chronologically before other organs. We used Picrosirius Red (PSR), a connective tissue stain specific for collagen I and III fibers, to evaluate ovarian fibrosis. Using bright-field, epifluorescence, confocal and polarized light microscopy, we validated the specific staining of highly ordered PSR-stained fibers in the ovary. We next examined ovarian PSR staining in two mouse strains (CD1 and CB6F1) across an aging continuum and found that PSR staining was minimal in ovaries from reproductively young adult animals, increased in distinct foci in animals of mid-to-advanced reproductive age, and was prominent throughout the stroma of the oldest animals. Consistent with fibrosis, there was a reproductive age-associated increase in ovarian hydroxyproline content. We also observed a unique population of multinucleated macrophage giant cells, which are associated with chronic inflammation, within the ovarian stroma exclusively in reproductively old mice. In fact, several genes central to inflammation had significantly higher levels of expression in ovaries from reproductively old mice relative to young mice. These results establish fibrosis as an early hallmark of the aging ovarian stroma, and this altered microenvironment may contribute to the age-associated decline in gamete quality.
© 2016 Society for Reproduction and Fertility.
Figures





References
-
- Alves A, Gritsch K, Sirieix C, Drevon-Gaillot E, Bayon Y, Clermont G, Boutrand JP, Grosgogeat B. Computerized histomorphometric study of the splenic collagen polymorphism: A control-tissue for polarization microscopy. Microsc Res Tech. 2015;78:900–907. - PubMed
-
- Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25:231–244. - PubMed
-
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–2623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

