Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine
- PMID: 27491926
- PMCID: PMC5311110
- DOI: 10.1002/anie.201606181
Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine
Abstract
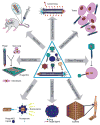
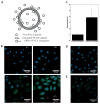
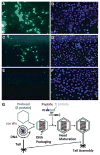
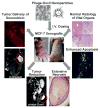
Both lytic and temperate bacteriophages (phages) can be applied in nanomedicine, in particular, as nanoprobes for precise disease diagnosis and nanotherapeutics for targeted disease treatment. Since phages are bacteria-specific viruses, they do not naturally infect eukaryotic cells and are not toxic to them. They can be genetically engineered to target nanoparticles, cells, tissues, and organs, and can also be modified with functional abiotic nanomaterials for disease diagnosis and treatment. This Review will summarize the current use of phage structures in many aspects of precision nanomedicine, including ultrasensitive biomarker detection, enhanced bioimaging for disease diagnosis, targeted drug and gene delivery, directed stem cell differentiation, accelerated tissue formation, effective vaccination, and nanotherapeutics for targeted disease treatment. We will also propose future directions in the area of phage-based nanomedicines, and discuss the state of phage-based clinical trials.
Keywords: biosensing; nanomedicine; phages; probes; therapeutics.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures











References
-
- d’Herelle F. C R Acad Sci Paris. 1917;165:373–375.
-
- Mao CB. In: Brenner’s Encyclopedia of Genetics. Stanley M, Kelly H, editors. Vol. 1. Academic Press; San Diego: 2013. pp. 39–41.
-
- Yosef I, Manor M, Kiro R, Qimron U. Proc Natl Acad Sci USA. 2015;112:7267–7272. - PMC - PubMed
- Marraffini L. FASEB J. 2016;30:107.101.
- Yosef I, Manor M, Qimron U. Bacteriophage. 2016;6:e1096996. - PMC - PubMed
- Villion M, Moineau S. Nature. 2013;494:433–434. - PubMed
- Verbeken G, Pirnay J, Lavigne R, Ceulemans C, De Vos D. J Microb Biochem Technol. 2016;8:021–024.
- Reardon S. Nature. 2014;510:15–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

