Plasmid pPCP1-derived sRNA HmsA promotes biofilm formation of Yersinia pestis
- PMID: 27492011
- PMCID: PMC4973556
- DOI: 10.1186/s12866-016-0793-5
Plasmid pPCP1-derived sRNA HmsA promotes biofilm formation of Yersinia pestis
Abstract
Background: The ability of Yersinia pestis to form a biofilm is an important characteristic in flea transmission of this pathogen. Y. pestis laterally acquired two plasmids (pPCP1and pMT1) and the ability to form biofilms when it evolved from Yersinia pseudotuberculosis. Small regulatory RNAs (sRNAs) are thought to play a crucial role in the processes of biofilm formation and pathogenesis.
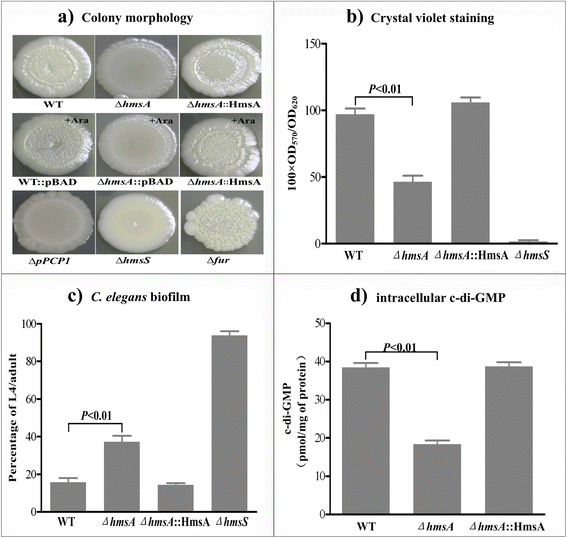
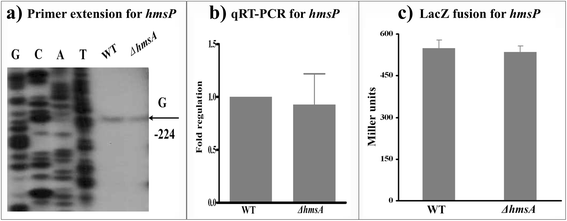
Results: A pPCP1-derived sRNA HmsA (also known as sR084) was found to contribute to the enhanced biofilm formation phenotype of Y. pestis. The concentration of c-di-GMP was significantly reduced upon deletion of the hmsA gene in Y. pestis. The abundance of mRNA transcripts determining exopolysaccharide production, crucial for biofilm formation, was measured by primer extension, RT-PCR and lacZ transcriptional fusion assays in the wild-type and hmsA mutant strains. HmsA positively regulated biofilm synthesis-associated genes (hmsHFRS, hmsT and hmsCDE), but had no regulatory effect on the biofilm degradation-associated gene hmsP. Interestingly, the recently identified biofilm activator sRNA, HmsB, was rapidly degraded in the hmsA deletion mutant. Two genes (rovM and rovA) functioning as biofilm regulators were also found to be regulated by HmsA, whose regulatory effects were consistent with the HmsA-mediated biofilm phenotype.
Conclusion: HmsA potentially functions as an activator of biofilm formation in Y. pestis, implying that sRNAs encoded on the laterally acquired plasmids might be involved in the chromosome-based regulatory networks implicated in Y. pestis-specific physiological processes.
Figures
References
-
- Erickson DL, Jarrett CO, Wren BW, Hinnebusch BJ. Serotype differences and lack of biofilm formation characterize Yersinia pseudotuberculosis infection of the Xenopsylla cheopis flea vector of Yersinia pestis. J Bacteriol. 2006;188(3):1113–9. doi: 10.1128/JB.188.3.1113-1119.2006. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

