Preterm infant gut microbiota affects intestinal epithelial development in a humanized microbiome gnotobiotic mouse model
- PMID: 27492329
- PMCID: PMC5076002
- DOI: 10.1152/ajpgi.00022.2016
Preterm infant gut microbiota affects intestinal epithelial development in a humanized microbiome gnotobiotic mouse model
Abstract
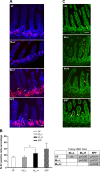
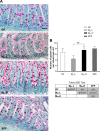
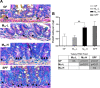
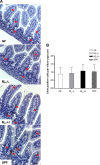
Development of the infant small intestine is influenced by bacterial colonization. To promote establishment of optimal microbial communities in preterm infants, knowledge of the beneficial functions of the early gut microbiota on intestinal development is needed. The purpose of this study was to investigate the impact of early preterm infant microbiota on host gut development using a gnotobiotic mouse model. Histological assessment of intestinal development was performed. The differentiation of four epithelial cell lineages (enterocytes, goblet cells, Paneth cells, enteroendocrine cells) and tight junction (TJ) formation was examined. Using weight gain as a surrogate marker for health, we found that early microbiota from a preterm infant with normal weight gain (MPI-H) induced increased villus height and crypt depth, increased cell proliferation, increased numbers of goblet cells and Paneth cells, and enhanced TJs compared with the changes induced by early microbiota from a poor weight gain preterm infant (MPI-L). Laser capture microdissection (LCM) plus qRT-PCR further revealed, in MPI-H mice, a higher expression of stem cell marker Lgr5 and Paneth cell markers Lyz1 and Cryptdin5 in crypt populations, along with higher expression of the goblet cell and mature enterocyte marker Muc3 in villus populations. In contrast, MPI-L microbiota failed to induce the aforementioned changes and presented intestinal characteristics comparable to a germ-free host. Our data demonstrate that microbial communities have differential effects on intestinal development. Future studies to identify pioneer settlers in neonatal microbial communities necessary to induce maturation may provide new insights for preterm infant microbial ecosystem therapeutics.
Keywords: development; gnotobiotic; gut; microbiota; preterm.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest 12: 355–364, 1963. - PubMed
-
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103, 2009. - PMC - PubMed
-
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

