Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys
- PMID: 27492477
- PMCID: PMC5237380
- DOI: 10.1126/science.aah6157
Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys
Abstract
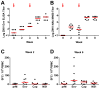
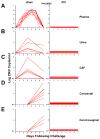
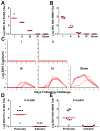
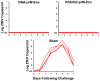
Zika virus (ZIKV) is responsible for a major ongoing epidemic in the Americas and has been causally associated with fetal microcephaly. The development of a safe and effective ZIKV vaccine is therefore an urgent global health priority. Here we demonstrate that three different vaccine platforms protect against ZIKV challenge in rhesus monkeys. A purified inactivated virus vaccine induced ZIKV-specific neutralizing antibodies and completely protected monkeys against ZIKV strains from both Brazil and Puerto Rico. Purified immunoglobulin from vaccinated monkeys also conferred passive protection in adoptive transfer studies. A plasmid DNA vaccine and a single-shot recombinant rhesus adenovirus serotype 52 vector vaccine, both expressing ZIKV premembrane and envelope, also elicited neutralizing antibodies and completely protected monkeys against ZIKV challenge. These data support the rapid clinical development of ZIKV vaccines for humans.
Copyright © 2016, American Association for the Advancement of Science.
Figures


Comment in
-
Zika vaccine trials.Science. 2016 Sep 9;353(6304):1094-5. doi: 10.1126/science.aai8126. Science. 2016. PMID: 27609872 No abstract available.
References
-
- Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016 Feb 18;374:601. - PubMed
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016 Apr 21;374:1552. - PubMed
-
- Mlakar J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016 Mar 10;374:951. - PubMed
-
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016 May 19;374:1981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

