Human native kappa opioid receptor functions not predicted by recombinant receptors: Implications for drug design
- PMID: 27492592
- PMCID: PMC4974614
- DOI: 10.1038/srep30797
Human native kappa opioid receptor functions not predicted by recombinant receptors: Implications for drug design
Abstract
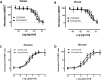
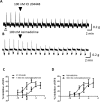
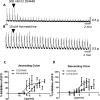
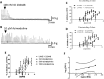
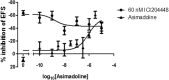
If activation of recombinant G protein-coupled receptors in host cells (by drugs or other ligands) has predictive value, similar data must be obtained with native receptors naturally expressed in tissues. Using mouse and human recombinant κ opioid receptors transfected into a host cell, two selectively-acting compounds (ICI204448, asimadoline) equi-effectively activated both receptors, assessed by measuring two different cell signalling pathways which were equally affected without evidence of bias. In mouse intestine, naturally expressing κ receptors within its nervous system, both compounds also equi-effectively activated the receptor, inhibiting nerve-mediated muscle contraction. However, whereas ICI204448 acted similarly in human intestine, where κ receptors are again expressed within its nervous system, asimadoline was inhibitory only at very high concentrations; instead, low concentrations of asimadoline reduced the activity of ICI204448. This demonstration of species-dependence in activation of native, not recombinant κ receptors may be explained by different mouse/human receptor structures affecting receptor expression and/or interactions with intracellular signalling pathways in native environments, to reveal differences in intrinsic efficacy between receptor agonists. These results have profound implications in drug design for κ and perhaps other receptors, in terms of recombinant-to-native receptor translation, species-dependency and possibly, a need to use human, therapeutically-relevant, not surrogate tissues.
Conflict of interest statement
G.J.S. received funding from Tioga to determine the effects of asimadoline and ICI204448 on cholinergically-mediated contractions of human and mouse isolated colon. G.J.S. currently receives funding from Takeda pharmaceuticals, The Dunhill Medical Trust, AgeUK and the BBSRC (Case award with GlaxoSmithKline) for unrelated studies. G.A.H. is now employed by Takeda Pharmaceuticals. The other authors declare no competing financial interests.
Figures

References
-
- Bourgeois C. et al. Synthesis and pharmacological evaluation of 5-pyrrolidinylquinoxalines as a novel class of peripherally restricted κ-opioid receptor agonists. J. Med. Chem. 57, 6845–6860 (2014). - PubMed
-
- Cheng M. F. et al. Discovery, structure-activity relationship studies, and anti-nociceptive effects of 1-phenyl-3,6,6-trimethyl-1,5,6,7-tetrahydro-4H-indazol-4-one as novel opioid receptor agonists. Bioorg. Med. Chem. 22, 4694–4703 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

