Impact of polymer-modified gold nanoparticles on brain endothelial cells: exclusion of endoplasmic reticulum stress as a potential risk factor
- PMID: 27492761
- PMCID: PMC5166978
- DOI: 10.1080/17435390.2016.1214761
Impact of polymer-modified gold nanoparticles on brain endothelial cells: exclusion of endoplasmic reticulum stress as a potential risk factor
Abstract
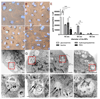
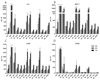
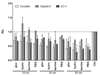
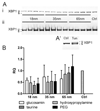
A library of polymer-coated gold nanoparticles (AuNPs) differing in size and surface modifications was examined for uptake and induction of cellular stress responses in the endoplasmic reticulum (ER stress) in human brain endothelial cells (hCMEC/D3). ER stress is known to affect the physiology of endothelial cells (ECs) and may lead to inflammation or apoptosis. Thus, even if applied at non-cytotoxic concentrations ER stress caused by nanoparticles should be prevented to reduce the risk of vascular diseases and negative effects on the integrity of barriers (e.g. blood-brain barrier). We exposed hCMEC/D3 to twelve different AuNPs (three sizes: 18, 35, and 65 nm, each with four surface-modifications) for various times and evaluated their effects on cytotoxicity, proinflammatory mediators, barrier functions and factors involved in ER stress. We demonstrated a time-dependent uptake of all AuNPs and no cytotoxicity for up to 72 h of exposure. Exposure to certain AuNPs resulted in a time-dependent increase in the proinflammatory markers IL-8, MCP-1, sVCAM, sICAM. However, none of the AuNPs induced an increase in expression of the chaperones and stress sensor proteins BiP and GRP94, respectively, or the transcription factors ATF4 and ATF6. Furthermore, no upregulation of the UPR stress sensor receptor PERK, no active splicing product of the transcription factor XBP1 and no upregulation of the transcription factor CHOP were detectable. In conclusion, the results of the present study indicate that effects of different-sized gold nanoparticles modified with various polymers were not related to the induction of ER stress in brain microvascular endothelial cells or led to apoptosis.
Keywords: BiP; blood-brain barrier; cell stress; tight junction proteins; unfolded protein response.
Conflict of interest statement
Declaration of Interest The authors declare that they have no competing interests.
Figures

Similar articles
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
Gene regulatory network of unfolded protein response genes in endoplasmic reticulum stress.Cell Stress Chaperones. 2013 Jan;18(1):11-23. doi: 10.1007/s12192-012-0351-5. Epub 2012 Jul 18. Cell Stress Chaperones. 2013. PMID: 22802018 Free PMC article.
-
Advanced glycation end-products induce endoplasmic reticulum stress in human aortic endothelial cells.Clin Chem Lab Med. 2014 Jan 1;52(1):151-60. doi: 10.1515/cclm-2012-0826. Clin Chem Lab Med. 2014. PMID: 23454718
-
Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70.Mol Pharmacol. 2007 Nov;72(5):1337-48. doi: 10.1124/mol.107.039164. Epub 2007 Aug 16. Mol Pharmacol. 2007. PMID: 17702888
-
Porcine brain microvessel endothelial cells show pro-inflammatory response to the size and composition of metallic nanoparticles.Drug Metab Rev. 2014 May;46(2):224-31. doi: 10.3109/03602532.2013.873450. Epub 2013 Dec 31. Drug Metab Rev. 2014. PMID: 24378227 Free PMC article. Review.
Cited by
-
Assessing cytotoxicity and endoplasmic reticulum stress in human blood-brain barrier cells due to silver and copper oxide nanoparticles.J Appl Genet. 2025 Feb;66(1):87-103. doi: 10.1007/s13353-024-00833-8. Epub 2024 Feb 9. J Appl Genet. 2025. PMID: 38332387 Free PMC article.
-
Time-Dependent Internalization of Polymer-Coated Silica Nanoparticles in Brain Endothelial Cells and Morphological and Functional Effects on the Blood-Brain Barrier.Int J Mol Sci. 2021 Feb 6;22(4):1657. doi: 10.3390/ijms22041657. Int J Mol Sci. 2021. PMID: 33562136 Free PMC article.
-
Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration.Sci Rep. 2019 Jun 4;9(1):8278. doi: 10.1038/s41598-019-44569-6. Sci Rep. 2019. PMID: 31164665 Free PMC article.
-
Carbon nanoparticles induce endoplasmic reticulum stress around blood vessels with accumulation of misfolded proteins in the developing brain of offspring.Sci Rep. 2020 Jun 22;10(1):10028. doi: 10.1038/s41598-020-66744-w. Sci Rep. 2020. PMID: 32572058 Free PMC article.
References
-
- Adachi Tetsuo, Teramachi Mayumi, Yasuda Hiroyuki, Kamiya Tetsuro, Hara Hirokazu. Contribution of p38 MAPK, NF-κB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Archives of biochemistry and biophysics. 2012;520(1):30–35. doi: 10.1016/j.abb.2012.01.014. S. - DOI - PubMed
-
- Bouzas Virginia, Haller Thomas, Hobi Nina, Felder Edward, Pastoriza-Santos Isabel, Pérez-Gil Jesús. Nontoxic impact of PEG-coated gold nanospheres on functional pulmonary surfactant-secreting alveolar type II cells. Nanotoxicology. 2014;8(8):813–823. doi: 10.3109/17435390.2013.829878. S. - DOI - PubMed
-
- Buttenschoen Klaus, Radermacher Peter, Bracht Hendrik. Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbeck's Archives of Surgery. 2010;395(6):597–605. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
