Novel Scoring Criteria for the Evaluation of Ocular Graft-versus-Host Disease in a Preclinical Allogeneic Hematopoietic Stem Cell Transplantation Animal Model
- PMID: 27492793
- PMCID: PMC5580988
- DOI: 10.1016/j.bbmt.2016.07.012
Novel Scoring Criteria for the Evaluation of Ocular Graft-versus-Host Disease in a Preclinical Allogeneic Hematopoietic Stem Cell Transplantation Animal Model
Abstract
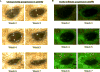
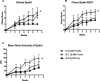
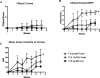
Ocular complications occur after transplantation in 60% to 90% of chronic graft-versus-host disease (GVHD) patients and significantly impair vision-related quality of life. Ocular surface inflammation and dry eye disease are the most common manifestations of ocular GVHD. Ocular GVHD can be viewed as an excellent preclinical model that can be studied to understand the immune pathogenesis of this common and debilitating disease. A limitation of this is that only a few experimental models mimic the ocular complications after hematopoietic stem cell transplantation (HSCT) and have focused on the acute GVHD process. To address this issue, we used a preclinical animal model developed by our group where ocular involvement was preceded by systemic GVHD to gain insight regarding the contributing immune mechanisms. Employing this "matched unrelated donor" model enabled the development of clinical scoring criteria, which readily identified different degrees of ocular pathology at both the ocular surface and adnexa, dependent on the level of conditioning before HSCT. As far as we are aware, we report for the first time that these clinical and immune responses occur not only on the ocular surface, but they also heavily involve the lid margin region. In total, the present study reports a preclinical scoring model that can be applied to animal models as investigators look to further explore GVHD's immunologic effects at the level of the ocular surface and eyelid adnexa compartments. We speculate that future studies will use this clinical scoring index in combination with what is recognized histologically and correlated with serum biomarkers identified in chronic/ocular GVHD.
Keywords: Allogeneic hematopoietic stem cell transplantation; Graft-versus-host disease (GVHD); Lacrimal gland; Ocular GVHT; Ocular adenexa; Ocular scoring.
Copyright © 2016 The American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
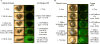
Similar articles
-
Ocular graft-versus-host disease after allogeneic stem cell transplantation.Cornea. 2010 Jul;29(7):758-63. doi: 10.1097/ICO.0b013e3181ca321c. Cornea. 2010. PMID: 20489577
-
[Clinical Signs of Ocular Graft-versus-Host Disease].Klin Monbl Augenheilkd. 2015 May;232(5):647-51. doi: 10.1055/s-0035-1545836. Epub 2015 May 19. Klin Monbl Augenheilkd. 2015. PMID: 25989033 Review. German.
-
A plethora of ocular surface manifestations in a multidisciplinary ocular graft-versus-host disease unit.Sci Rep. 2022 Sep 23;12(1):15926. doi: 10.1038/s41598-022-19990-z. Sci Rep. 2022. PMID: 36151252 Free PMC article.
-
Ocular graft-versus-host disease: a review.Surv Ophthalmol. 2013 May-Jun;58(3):233-51. doi: 10.1016/j.survophthal.2012.08.004. Epub 2013 Mar 27. Surv Ophthalmol. 2013. PMID: 23541042 Review.
-
Ocular findings after allogeneic hematopoietic stem cell transplantation.Ophthalmology. 2009 Sep;116(9):1624-9. doi: 10.1016/j.ophtha.2009.04.054. Ophthalmology. 2009. PMID: 19729097
Cited by
-
Positive Effects of Oral Antibiotic Administration in Murine Chronic Graft-Versus-Host Disease.Int J Mol Sci. 2021 Apr 3;22(7):3745. doi: 10.3390/ijms22073745. Int J Mol Sci. 2021. PMID: 33916809 Free PMC article.
-
Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease.Int J Mol Sci. 2021 Dec 24;23(1):184. doi: 10.3390/ijms23010184. Int J Mol Sci. 2021. PMID: 35008621 Free PMC article.
-
"Smart Eye Camera": An innovative technique to evaluate tear film breakup time in a murine dry eye disease model.PLoS One. 2019 May 9;14(5):e0215130. doi: 10.1371/journal.pone.0215130. eCollection 2019. PLoS One. 2019. PMID: 31071120 Free PMC article.
-
Complement and CD4+ T cells drive context-specific corneal sensory neuropathy.Elife. 2019 Aug 15;8:e48378. doi: 10.7554/eLife.48378. Elife. 2019. PMID: 31414985 Free PMC article.
-
Murine models of graft versus host disease (GVHD): Focus on ocular GVHD.Ocul Surf. 2023 Oct;30:179-186. doi: 10.1016/j.jtos.2023.09.006. Epub 2023 Sep 22. Ocul Surf. 2023. PMID: 37742740 Free PMC article. Review.
References
-
- Franklin RM, Kenyon KR, Tutschka PJ, Saral R, Green WR, Santos GW. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983;90(1):4–13. - PubMed
-
- Riemens A, te Boome L, Imhof S, Kuball J, Rothova A. Current insights into ocular graft-versus-host disease. Curr Opin Ophthalmol. 2010;21(6):485–94. - PubMed
-
- Hirst LW, Jabs DA, Tutschka PJ, Green WR, Santos GW. The eye in bone marrow transplantation. I. Clinical study. Arch Ophthalmol. 1983;101(4):580–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

