Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6
- PMID: 27493064
- PMCID: PMC4974504
- DOI: 10.1038/srep30282
Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6
Abstract
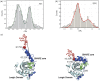
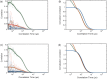
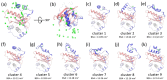
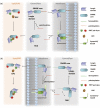
Cellular informational and metabolic processes are propagated with specific membrane fusions governed by soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE). SNARE protein Ykt6 is highly expressed in brain neurons and plays a critical role in the membrane-trafficking process. Studies suggested that Ykt6 undergoes a conformational change at the interface between its longin domain and the SNARE core. In this work, we study the conformational state distributions and dynamics of rat Ykt6 by means of single-molecule Förster Resonance Energy Transfer (smFRET) and Fluorescence Cross-Correlation Spectroscopy (FCCS). We observed that intramolecular conformational dynamics between longin domain and SNARE core occurred at the timescale ~200 μs. Furthermore, this dynamics can be regulated and even eliminated by the presence of lipid dodecylphoshpocholine (DPC). Our molecular dynamic (MD) simulations have shown that, the SNARE core exhibits a flexible structure while the longin domain retains relatively stable in apo state. Combining single molecule experiments and theoretical MD simulations, we are the first to provide a quantitative dynamics of Ykt6 and explain the functional conformational change from a qualitative point of view.
Figures

References
-
- Söllner T. et al.. SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 (1993). - PubMed
-
- McNew J. A. et al.. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 (2000). - PubMed
-
- Hu C. et al.. Fusion of cells by flipped SNAREs. Science 300, 1745–1749 (2003). - PubMed
-
- Hay J. C. SNARE complex structure and function. Exp. Cell Res. 271, 10–21 (2001). - PubMed
-
- Ungar D. & Hughson F. M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493–517 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

