Electrophysiological characteristics of feeding-related neurons after taste avoidance Pavlovian conditioning in Lymnaea stagnalis
- PMID: 27493506
- PMCID: PMC4629664
- DOI: 10.2142/biophysics.10.121
Electrophysiological characteristics of feeding-related neurons after taste avoidance Pavlovian conditioning in Lymnaea stagnalis
Abstract
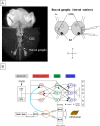
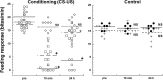
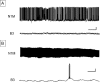
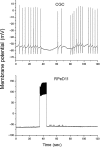
Taste avoidance conditioning (TAC) was carried out on the pond snail, Lymnaea stagnalis. The conditional stimulus (CS) was sucrose which elicits feeding behavior; while the unconditional stimulus (US) was a tactile stimulus to the head which causes feeding to be suppressed. The neuronal circuit that drives feeding behavior in Lymnaea is well worked out. We therefore compared the physiological characteristics on 3 classes of neurons involved with feeding behavior especially in response to the CS in conditioned vs. control snails. The cerebral giant cell (CGC) modulates feeding behavior, N1 medial neuron (N1M) is one of the central pattern generator neurons that organizes feeding behavior, while B3 is a motor neuron active during the rasp phase of feeding. We found the resting membrane potential in CGC was hyperpolarized significantly in conditioned snails but impulse activity remained the same between conditioned vs. control snails. There was, however, a significant increase in spontaneous activity and a significant depolarization of N1M's resting membrane potential in conditioned snails. These changes in N1M activity as a result of training are thought to be due to withdrawal interneuron RPeD11 altering the activity of the CGCs. Finally, in B3 there was: 1) a significant decrease in the amplitude and the frequency of the post-synaptic potentials; 2) a significant hyperpolarization of resting membrane potential in conditioned snails; and 3) a disappearance of bursting activity typically initiated by the CS. These neuronal modifications are consistent with the behavioral phenotype elicited by the CS following conditioning.
Keywords: Lymnaea; central pattern generator; modulatory neuron; motor neuron; taste avoidance conditioning.
Figures




Similar articles
-
Memory trace in feeding neural circuitry underlying conditioned taste aversion in Lymnaea.PLoS One. 2012;7(8):e43151. doi: 10.1371/journal.pone.0043151. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900097 Free PMC article.
-
Central pattern generator interneurons are targets for the modulatory serotonergic cerebral giant cells in the feeding system of Lymnaea.J Neurophysiol. 1996 Jan;75(1):11-25. doi: 10.1152/jn.1996.75.1.11. J Neurophysiol. 1996. PMID: 8822538
-
Enhancement of an inhibitory input to the feeding central pattern generator in Lymnaea stagnalis during conditioned taste-aversion learning.Neurosci Lett. 1997 Jul 25;230(3):179-82. doi: 10.1016/s0304-3940(97)00507-7. Neurosci Lett. 1997. PMID: 9272690
-
A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea.Learn Mem. 2000 May-Jun;7(3):124-31. doi: 10.1101/lm.7.3.124. Learn Mem. 2000. PMID: 10837501 Review.
-
Another Example of Conditioned Taste Aversion: Case of Snails.Biology (Basel). 2020 Nov 26;9(12):422. doi: 10.3390/biology9120422. Biology (Basel). 2020. PMID: 33256267 Free PMC article. Review.
Cited by
-
Unique morphology and photoperiodically regulated activity of neurosecretory canopy cells in the pond snail Lymnaea stagnalis.Cell Tissue Res. 2023 Sep;393(3):547-558. doi: 10.1007/s00441-023-03799-x. Epub 2023 Jul 7. Cell Tissue Res. 2023. PMID: 37418027 Free PMC article.
-
Function of insulin in snail brain in associative learning.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Oct;201(10):969-81. doi: 10.1007/s00359-015-1032-5. Epub 2015 Aug 2. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 26233474 Review.
-
Effects of serotonin on the heartbeat of pond snails in a hunger state.Biophysics (Nagoya-shi). 2015 Jan 5;11:1-5. doi: 10.2142/biophysics.11.1. eCollection 2015. Biophysics (Nagoya-shi). 2015. PMID: 27493507 Free PMC article.
References
-
- Benjamin PR, Staras K, Kemenes G. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea. Learn Mem. 2000;7:124–131. - PubMed
-
- Jones N, Kemenes G, Benjamin PR. Selective expression of electrical correlates of differential appetitive classical conditioning in a feeding network. J Neurophysiol. 2001;85:89–97. - PubMed
-
- Staras K, Kemenes G, Benjamin PR. Electrophysiological and behavioral analysis of lip touch as a component of the food stimulus in the snail Lymnaea. J Neurophysiol. 1999;81:1261–1273. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
