"Decoding" Angiogenesis: New Facets Controlling Endothelial Cell Behavior
- PMID: 27493632
- PMCID: PMC4954849
- DOI: 10.3389/fphys.2016.00306
"Decoding" Angiogenesis: New Facets Controlling Endothelial Cell Behavior
Abstract
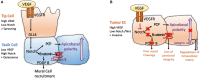
Angiogenesis, the formation of new blood vessels, is a unique and crucial biological process occurring during both development and adulthood. A better understanding of the mechanisms that regulates such process is mandatory to intervene in pathophysiological conditions. Here we highlight some recent argument on new players that are critical in endothelial cells, by summarizing novel discoveries that regulate notorious vascular pathways such as Vascular Endothelial Growth Factor (VEGF), Notch and Planar Cell Polarity (PCP), and by discussing more recent findings that put metabolism, redox signaling and hemodynamic forces as novel unforeseen facets in angiogenesis. These new aspects, that critically regulate angiogenesis and vascular homeostasis in health and diseased, represent unforeseen new ground to develop anti-angiogenic therapies.
Keywords: Notch signaling pathway; angiogenesis; flow dynamics; metabolism; redox signaling.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

