Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview
- PMID: 27493788
- PMCID: PMC4964220
- DOI: 10.1136/rmdopen-2015-000239
Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview
Abstract
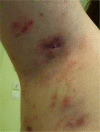
Paradoxical adverse events (PAEs) have been reported during biological treatment for chronic immune-mediated diseases. PAEs are defined as the occurrence during biological agent therapy of a pathological condition that usually responds to this class of drug. A wide range of PAEs have been reported including dermatological, intestinal and ophthalmic conditions, mainly with antitumour necrosis factor α (TNF-α) agents. True PAEs include psoriasis, Crohn's disease and hidradenitis suppurativa. Other PAEs may be qualified as borderline and include uveitis, scleritis, sarcoidosis and other granulomatous diseases (granuloma annulare, interstitial granulomatous dermatitis), vasculitis, vitiligo and alopecia areata. Proposed hypotheses to explain these PAEs include an imbalance in cytokine production, the differential immunological properties between the monoclonal antibodies and TNF-α soluble receptor, an unopposed type I interferon production and a shift towards a Th1/Th2 profile. Data from registries suggest that the risk for paradoxical psoriasis is low and non-significant. We discuss management of these PAEs, which depends on the type and severity of the adverse events, pre-existing treated conditions and the possibility of alternative therapeutic options for the underlying disease. Paradoxical adverse events are not restricted to anti-TNF-α agents and close surveillance of new available biological drugs (anti-interleukin-17/23, anti-integrin) is warranted in order to detect the occurrence of new or as yet undescribed events.
Keywords: Anti-TNF; DMARDs (biologic); Sarcoidosis; Treatment.
Figures





References
-
- Joyau C, Veyrac G, Dixneuf V et al. . Anti-tumour necrosis factor alpha therapy and increased risk of de novo psoriasis: is it really a paradoxical side effect? Clin Exp Rheumatol 2012;30:700–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
