Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units
- PMID: 27493991
- PMCID: PMC4972469
- DOI: 10.1126/sciadv.1501429
Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units
Abstract
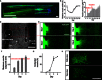
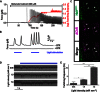
Motor units are the fundamental elements responsible for muscle movement. They are formed by lower motor neurons and their muscle targets, synapsed via neuromuscular junctions (NMJs). The loss of NMJs in neurodegenerative disorders (such as amyotrophic lateral sclerosis or spinal muscle atrophy) or as a result of traumatic injuries affects millions of lives each year. Developing in vitro assays that closely recapitulate the physiology of neuromuscular tissues is crucial to understand the formation and maturation of NMJs, as well as to help unravel the mechanisms leading to their degeneration and repair. We present a microfluidic platform designed to coculture myoblast-derived muscle strips and motor neurons differentiated from mouse embryonic stem cells (ESCs) within a three-dimensional (3D) hydrogel. The device geometry mimics the spinal cord-limb physical separation by compartmentalizing the two cell types, which also facilitates the observation of 3D neurite outgrowth and remote muscle innervation. Moreover, the use of compliant pillars as anchors for muscle strips provides a quantitative functional readout of force generation. Finally, photosensitizing the ESC provides a pool of source cells that can be differentiated into optically excitable motor neurons, allowing for spatiodynamic, versatile, and noninvasive in vitro control of the motor units.
Keywords: Microfluidics; neuromuscular junctions; optogenetics; tissue engineering.
Figures




References
-
- Olesen J., Gustavsson A., Svensson M., Wittchen H.-U., Jönsson B. CDBE2010 study group European Brain Council , The economic cost of brain disorders in Europe. Eur. J. Neurol. 19, 155–162 (2012). - PubMed
-
- Sanes J. R., Lichtman J. W., Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

